Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leucine--tRNA ligase, cytoplasmic
Ligand
BDBM50075069
Substrate
n/a
Meas. Tech.
ChEBML_98551
IC50
730±n/a nM
Citation
 Yu, XY; Hill, JM; Yu, G; Wang, W; Kluge, AF; Wendler, P; Gallant, P Synthesis and structure-activity relationships of a series of novel thiazoles as inhibitors of aminoacyl-tRNA synthetases. Bioorg Med Chem Lett 9:375-80 (1999) [PubMed] Article
Yu, XY; Hill, JM; Yu, G; Wang, W; Kluge, AF; Wendler, P; Gallant, P Synthesis and structure-activity relationships of a series of novel thiazoles as inhibitors of aminoacyl-tRNA synthetases. Bioorg Med Chem Lett 9:375-80 (1999) [PubMed] Article More Info.:
Target
Name:
Leucine--tRNA ligase, cytoplasmic
Synonyms:
KIAA1352 | LARS | LARS1 | Leucyl-tRNA synthetase | SYLC_HUMAN
Type:
PROTEIN
Mol. Mass.:
134475.20
Organism:
Homo sapiens (Human)
Description:
ChEMBL_98544
Residue:
1176
Sequence:
MAERKGTAKVDFLKKIEKEIQQKWDTERVFEVNASNLEKQTSKGKYFVTFPYPYMNGRLHLGHTFSLSKCEFAVGYQRLKGKCCLFPFGLHCTGMPIKACADKLKREIELYGCPPDFPDEEEEEEETSVKTEDIIIKDKAKGKKSKAAAKAGSSKYQWGIMKSLGLSDEEIVKFSEAEHWLDYFPPLAIQDLKRMGLKVDWRRSFITTDVNPYYDSFVRWQFLTLRERNKIKFGKRYTIYSPKDGQPCMDHDRQTGEGVGPQEYTLLKLKVLEPYPSKLSGLKGKNIFLVAATLRPETMFGQTNCWVRPDMKYIGFETVNGDIFICTQKAARNMSYQGFTKDNGVVPVVKELMGEEILGASLSAPLTSYKVIYVLPMLTIKEDKGTGVVTSVPSDSPDDIAALRDLKKKQALRAKYGIRDDMVLPFEPVPVIEIPGFGNLSAVTICDELKIQSQNDREKLAEAKEKIYLKGFYEGIMLVDGFKGQKVQDVKKTIQKKMIDAGDALIYMEPEKQVMSRSSDECVVALCDQWYLDYGEENWKKQTSQCLKNLETFCEETRRNFEATLGWLQEHACSRTYGLGTHLPWDEQWLIESLSDSTIYMAFYTVAHLLQGGNLHGQAESPLGIRPQQMTKEVWDYVFFKEAPFPKTQIAKEKLDQLKQEFEFWYPVDLRVSGKDLVPNHLSYYLYNHVAMWPEQSDKWPTAVRANGHLLLNSEKMSKSTGNFLTLTQAIDKFSADGMRLALADAGDTVEDANFVEAMADAGILRLYTWVEWVKEMVANWDSLRSGPASTFNDRVFASELNAGIIKTDQNYEKMMFKEALKTGFFEFQAAKDKYRELAVEGMHRELVFRFIEVQTLLLAPFCPHLCEHIWTLLGKPDSIMNASWPVAGPVNEVLIHSSQYLMEVTHDLRLRLKNYMMPAKGKKTDKQPLQKPSHCTIYVAKNYPPWQHTTLSVLRKHFEANNGKLPDNKVIASELGSMPELKKYMKKVMPFVAMIKENLEKMGPRILDLQLEFDEKAVLMENIVYLTNSLELEHIEVKFASEAEDKIREDCCPGKPLNVFRIEPGVSVSLVNPQPSNGHFSTKIEIRQGDNCDSIIRRLMKMNRGIKDLSKVKLMRFDDPLLGPRRVPVLGKEYTEKTPISEHAVFNVDLMSKKIHLTENGIRVDIGDTIIYLVH
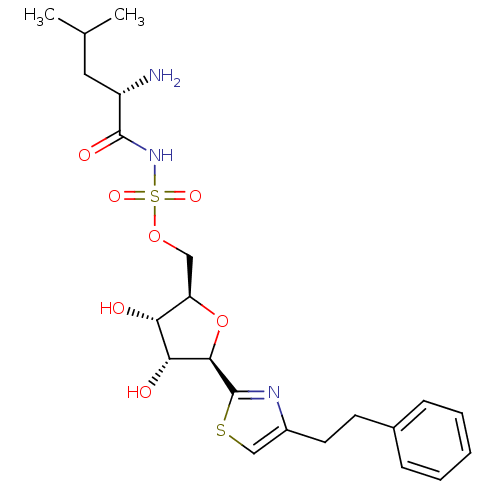
Inhibitor
Name:
BDBM50075069
Synonyms:
((S)-2-Amino-4-methyl-pentanoyl)-sulfamic acid (2R,3S,4R,5R)-3,4-dihydroxy-5-(4-phenethyl-thiazol-2-yl)-tetrahydro-furan-2-ylmethyl ester | CHEMBL332104
Type:
Small organic molecule
Emp. Form.:
C22H31N3O7S2
Mol. Mass.:
513.627
SMILES:
CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)c1nc(CCc2ccccc2)cs1