Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM50029616
Substrate
n/a
Meas. Tech.
ChEMBL_44005 (CHEMBL655571)
IC50
70.0±n/a nM
Citation
 Song, Y; Connor, DT; Sercel, AD; Sorenson, RJ; Doubleday, R; Unangst, PC; Roth, BD; Beylin, VG; Gilbertsen, RB; Chan, K; Schrier, DJ; Guglietta, A; Bornemeier, DA; Dyer, RD Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-thiadiazole series. J Med Chem 42:1161-9 (1999) [PubMed] Article
Song, Y; Connor, DT; Sercel, AD; Sorenson, RJ; Doubleday, R; Unangst, PC; Roth, BD; Beylin, VG; Gilbertsen, RB; Chan, K; Schrier, DJ; Guglietta, A; Bornemeier, DA; Dyer, RD Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-thiadiazole series. J Med Chem 42:1161-9 (1999) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
Cox-2 | Cox2 | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2) | Glucocorticoid-regulated inflammatory cyclooxygenase | Gripghs | Macrophage activation-associated marker protein P71/73 | PES-2 | PGH synthase 2 | PGH2_MOUSE | PGHS-2 | PHS II | Pghs-b | Prostaglandin G/H synthase (cyclooxygenase) | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | TIS10 protein | Tis10
Type:
Protein
Mol. Mass.:
69020.39
Organism:
Mus musculus (Mouse)
Description:
Q05769
Residue:
604
Sequence:
MLFRAVLLCAALGLSQAANPCCSNPCQNRGECMSTGFDQYKCDCTRTGFYGENCTTPEFLTRIKLLLKPTPNTVHYILTHFKGVWNIVNNIPFLRSLIMKYVLTSRSYLIDSPPTYNVHYGYKSWEAFSNLSYYTRALPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPGFTRGLGHGVDLNHIYGETLDRQHKLRLFKDGKLKYQVIGGEVYPPTVKDTQVEMIYPPHIPENLQFAVGQEVFGLVPGLMMYATIWLREHNRVCDILKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQNRIASEFNTLYHWHPLLPDTFNIEDQEYSFKQFLYNNSILLEHGLTQFVESFTRQIAGRVAGGRNVPIAVQAVAKASIDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKALYSDIDVMELYPALLVEKPRPDAIFGETMVELGAPFSLKGLMGNPICSPQYWKPSTFGGEVGFKIINTASIQSLICNNVKGCPFTSFNVQDPQPTKTATINASASHSRLDDINPTVLIKRRSTEL
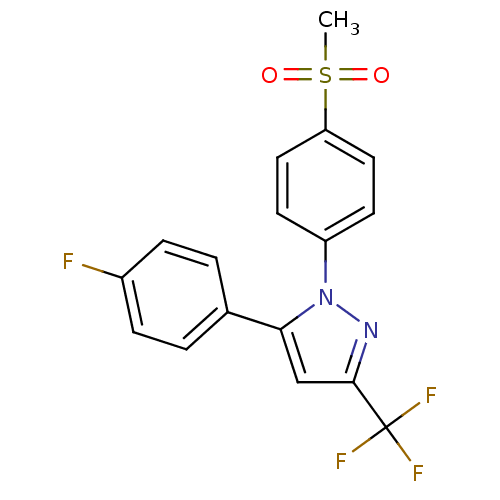
Inhibitor
Name:
BDBM50029616
Synonyms:
5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3-trifluoromethyl-1H-pyrazole | 5-(4-fluorophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(trifluoromethyl)-1H-pyrazole | CHEMBL274990 | SC-58125 | SC58125
Type:
Small organic molecule
Emp. Form.:
C17H12F4N2O2S
Mol. Mass.:
384.348
SMILES:
CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F