Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lactoylglutathione lyase
Ligand
BDBM16452
Substrate
n/a
Meas. Tech.
ChEMBL_1901794 (CHEMBL4404016)
Ki
1.2±n/a nM
Citation
More Info.:
Target
Name:
Lactoylglutathione lyase
Synonyms:
Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase
Type:
Enzyme
Mol. Mass.:
20772.95
Organism:
Homo sapiens (Human)
Description:
Q04760
Residue:
184
Sequence:
MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQKCDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNSDPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKMATLM
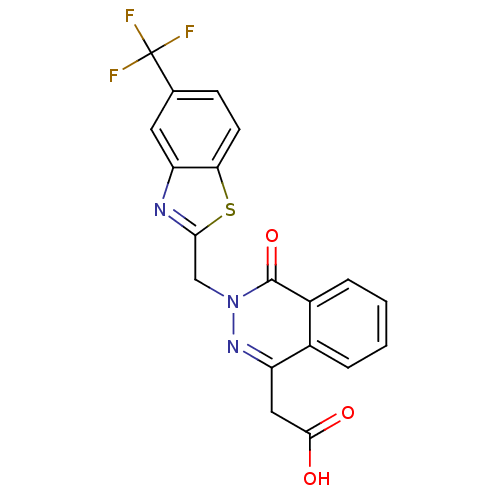
Inhibitor
Name:
BDBM16452
Synonyms:
(4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-yl]methyl}-3,4-dihydrophthalazin-1-yl)acetic acid | 2-(4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-yl]methyl}-3,4-dihydrophthalazin-1-yl)acetic acid | Alond | CHEMBL10372 | Xedia | Zopolrestat
Type:
Small organic molecule
Emp. Form.:
C19H12F3N3O3S
Mol. Mass.:
419.377
SMILES:
OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12