Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ribosomal protein S6 kinase alpha-1
Ligand
BDBM50528409
Substrate
n/a
Meas. Tech.
ChEMBL_1905011 (CHEMBL4407369)
IC50
32±n/a nM
Citation
 Dong, X; Zhan, W; Zhao, M; Che, J; Dai, X; Wu, Y; Xu, L; Zhou, Y; Zhao, Y; Tian, T; Cheng, G; Jin, Z; Li, J; Shao, Y; He, Q; Yang, B; Weng, Q; Hu, Y Discovery of 3,4,6-Trisubstituted Piperidine Derivatives as Orally Active, Low hERG Blocking Akt Inhibitors via Conformational Restriction and Structure-Based Design. J Med Chem 62:7264-7288 (2019) [PubMed] Article
Dong, X; Zhan, W; Zhao, M; Che, J; Dai, X; Wu, Y; Xu, L; Zhou, Y; Zhao, Y; Tian, T; Cheng, G; Jin, Z; Li, J; Shao, Y; He, Q; Yang, B; Weng, Q; Hu, Y Discovery of 3,4,6-Trisubstituted Piperidine Derivatives as Orally Active, Low hERG Blocking Akt Inhibitors via Conformational Restriction and Structure-Based Design. J Med Chem 62:7264-7288 (2019) [PubMed] Article More Info.:
Target
Name:
Ribosomal protein S6 kinase alpha-1
Synonyms:
90 kDa ribosomal protein S6 kinase 1 | KS6A1_HUMAN | MAP kinase-activated protein kinase 1a | MAPKAPK1A | RPS6KA1 | RPS6KA1(Kin.Dom.1 - N-terminal) | RPS6KA1(Kin.Dom.2 - C-terminal) | RSK-1 | RSK1 | Ribosomal S6 Kinase 1 (RSK-1) | Ribosomal S6 kinase 1 | Ribosomal S6 kinase 1 (RSK1) | Ribosomal protein S6 kinase alpha 1 | Ribosomal protein S6 kinase alpha-1 | Ribosomal protein S6 kinase alpha-1 (RSK1) | S6K-alpha 1 | p90-RSK 1 | p90RSK | p90S6K | pp90RSK1
Type:
Serine/threonine-protein kinase
Mol. Mass.:
82736.10
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
735
Sequence:
MPLAQLKEPWPLMELVPLDPENGQTSGEEAGLQPSKDEGVLKEISITHHVKAGSEKADPSHFELLKVLGQGSFGKVFLVRKVTRPDSGHLYAMKVLKKATLKVRDRVRTKMERDILADVNHPFVVKLHYAFQTEGKLYLILDFLRGGDLFTRLSKEVMFTEEDVKFYLAELALGLDHLHSLGIIYRDLKPENILLDEEGHIKLTDFGLSKEAIDHEKKAYSFCGTVEYMAPEVVNRQGHSHSADWWSYGVLMFEMLTGSLPFQGKDRKETMTLILKAKLGMPQFLSTEAQSLLRALFKRNPANRLGSGPDGAEEIKRHVFYSTIDWNKLYRREIKPPFKPAVAQPDDTFYFDTEFTSRTPKDSPGIPPSAGAHQLFRGFSFVATGLMEDDGKPRAPQAPLHSVVQQLHGKNLVFSDGYVVKETIGVGSYSECKRCVHKATNMEYAVKVIDKSKRDPSEEIEILLRYGQHPNIITLKDVYDDGKHVYLVTELMRGGELLDKILRQKFFSEREASFVLHTIGKTVEYLHSQGVVHRDLKPSNILYVDESGNPECLRICDFGFAKQLRAENGLLMTPCYTANFVAPEVLKRQGYDEGCDIWSLGILLYTMLAGYTPFANGPSDTPEEILTRIGSGKFTLSGGNWNTVSETAKDLVSKMLHVDPHQRLTAKQVLQHPWVTQKDKLPQSQLSHQDLQLVKGAMAATYSALNSSKPTPQLKPIESSILAQRRVRKLPSTTL
Inhibitor
Name:
BDBM50528409
Synonyms:
CHEMBL4441825
Type:
Small organic molecule
Emp. Form.:
C22H22Cl2F2N4O3
Mol. Mass.:
499.338
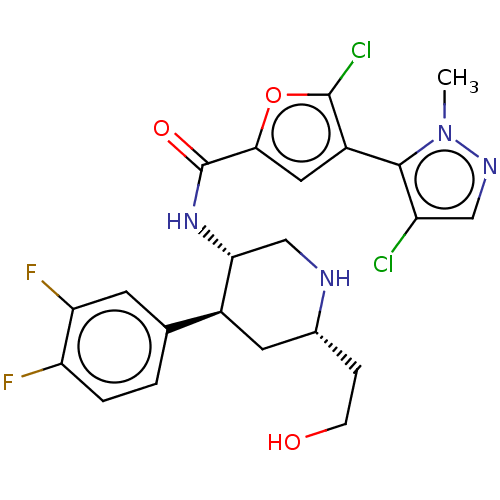
SMILES:
Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@@H]1CN[C@H](CCO)C[C@H]1c1ccc(F)c(F)c1 |r,wU:16.17,19.21,wD:24.27,(5.73,-5.85,;5.41,-7.35,;4,-7.98,;4.16,-9.51,;5.66,-9.83,;6.29,-11.24,;6.44,-8.5,;7.97,-8.34,;9,-9.49,;10.41,-8.86,;10.25,-7.33,;8.75,-7.01,;8.12,-5.6,;11.75,-9.64,;11.74,-11.18,;13.08,-8.87,;14.41,-9.65,;14.41,-11.18,;15.74,-11.96,;17.08,-11.19,;18.41,-11.96,;19.74,-11.2,;21.07,-11.97,;17.08,-9.64,;15.75,-8.88,;15.75,-7.35,;14.42,-6.58,;14.42,-5.03,;15.75,-4.26,;15.74,-2.72,;17.08,-5.03,;18.41,-4.25,;17.09,-6.58,)|