Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM22953
Substrate
n/a
Meas. Tech.
ChEMBL_43839 (CHEMBL658501)
IC50
250±n/a nM
Citation
 Kalgutkar, AS; Marnett, AB; Crews, BC; Remmel, RP; Marnett, LJ Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem 43:2860-70 (2000) [PubMed] Article
Kalgutkar, AS; Marnett, AB; Crews, BC; Remmel, RP; Marnett, LJ Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem 43:2860-70 (2000) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
COX2 | Cyclooxygenase | Cyclooxygenase 2 (COX-2) | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2 AA) | Cyclooxygenase-2 (COX-2 AEA) | Cyclooxygenase-2 (COX-2) | PGH synthase 2 | PGH2_HUMAN | PGHS-2 | PHS II | PTGS2 | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2
Type:
Enzyme
Mol. Mass.:
69003.89
Organism:
Homo sapiens (Human)
Description:
Recombinant Cox-2 provided by Cayman (Cayman Chemical Co.,Ann Arbor, MI).
Residue:
604
Sequence:
MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFLTRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADYGYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKYQIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCDVLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQNRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRVAGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEALYGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEVGFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKERSTEL
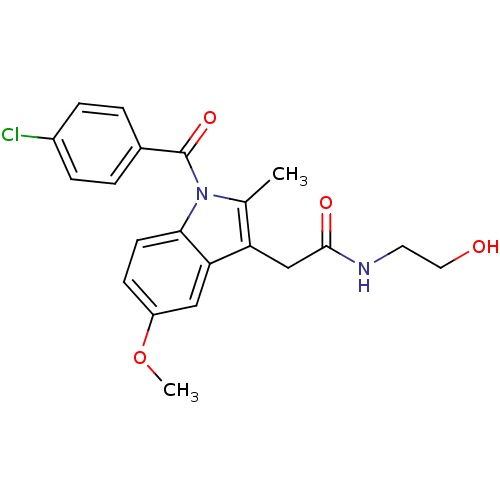
Inhibitor
Name:
BDBM22953
Synonyms:
2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}-N-(2-hydroxyethyl)acetamide | CHEMBL24415 | Indomethacin derivative, 6
Type:
Small organic molecule
Emp. Form.:
C21H21ClN2O4
Mol. Mass.:
400.855
SMILES:
COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCO)c2c1