Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Beta-1 adrenergic receptor
Ligand
BDBM50092645
Substrate
n/a
Meas. Tech.
ChEMBL_37375 (CHEMBL649965)
IC50
2300±n/a nM
Citation
 Mathvink, RJ; Tolman, JS; Chitty, D; Candelore, MR; Cascieri, MA; Colwell, LF; Deng, L; Feeney, WP; Forrest, MJ; Hom, GJ; MacIntyre, DE; Miller, RR; Stearns, RA; Tota, L; Wyvratt, MJ; Fisher, MH; Weber, AE Discovery of a potent, orally bioavailable beta(3) adrenergic receptor agonist, (R)-N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4 -[4-(trifluoromethyl)phenyl]thiazol-2-yl]benzenesulfonamide. J Med Chem 43:3832-6 (2000) [PubMed] Article
Mathvink, RJ; Tolman, JS; Chitty, D; Candelore, MR; Cascieri, MA; Colwell, LF; Deng, L; Feeney, WP; Forrest, MJ; Hom, GJ; MacIntyre, DE; Miller, RR; Stearns, RA; Tota, L; Wyvratt, MJ; Fisher, MH; Weber, AE Discovery of a potent, orally bioavailable beta(3) adrenergic receptor agonist, (R)-N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4 -[4-(trifluoromethyl)phenyl]thiazol-2-yl]benzenesulfonamide. J Med Chem 43:3832-6 (2000) [PubMed] Article More Info.:
Target
Name:
Beta-1 adrenergic receptor
Synonyms:
ADRB1 | ADRB1R | ADRB1_HUMAN | B1AR | Beta-1 adrenoceptor | Beta-1 adrenoreceptor | adrenergic Beta1
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
51338.40
Organism:
Homo sapiens (Human)
Description:
P08588
Residue:
477
Sequence:
MGAGVLVLGASEPGNLSSAAPLPDGAATAARLLVPASPPASLLPPASESPEPLSQQWTAGMGLLMALIVLLIVAGNVLVIVAIAKTPRLQTLTNLFIMSLASADLVMGLLVVPFGATIVVWGRWEYGSFFCELWTSVDVLCVTASIETLCVIALDRYLAITSPFRYQSLLTRARARGLVCTVWAISALVSFLPILMHWWRAESDEARRCYNDPKCCDFVTNRAYAIASSVVSFYVPLCIMAFVYLRVFREAQKQVKKIDSCERRFLGGPARPPSPSPSPVPAPAPPPGPPRPAAAAATAPLANGRAGKRRPSRLVALREQKALKTLGIIMGVFTLCWLPFFLANVVKAFHRELVPDRLFVFFNWLGYANSAFNPIIYCRSPDFRKAFQGLLCCARRAARRRHATHGDRPRASGCLARPGPPPSPGAASDDDDDDVVGATPPARLLEPWAGCNGGAAADSDSSLDEPCRPGFASESKV
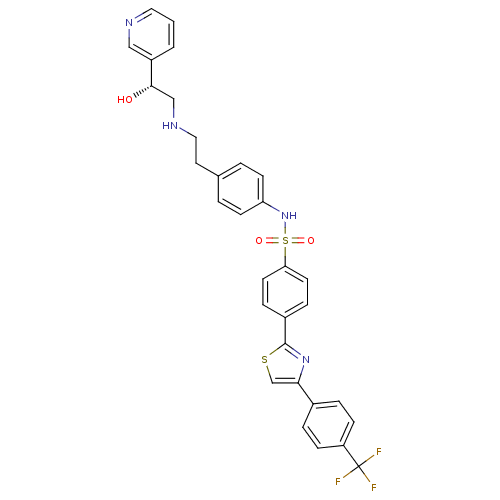
Inhibitor
Name:
BDBM50092645
Synonyms:
(R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)ethyl)phenyl)-4-(4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)benzenesulfonamide | CHEMBL111201 | L-796568 | N-{4-[2-((R)-2-Hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[4-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide | N-{4-[2-((R)-2-hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[3-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide | N-{4-[2-(2-Hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[4-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide
Type:
Small organic molecule
Emp. Form.:
C31H27F3N4O3S2
Mol. Mass.:
624.696
SMILES:
O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1