Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bifunctional purine biosynthesis protein ATIC
Ligand
BDBM50103596
Substrate
n/a
Meas. Tech.
ChEMBL_1988115 (CHEMBL4621662)
Ki
22000±n/a nM
Citation
 Duff, MR; Gabel, SA; Pedersen, LC; DeRose, EF; Krahn, JM; Howell, EE; London, RE The Structural Basis for Nonsteroidal Anti-Inflammatory Drug Inhibition of Human Dihydrofolate Reductase. J Med Chem 63:8314-8324 (2020) [PubMed] Article
Duff, MR; Gabel, SA; Pedersen, LC; DeRose, EF; Krahn, JM; Howell, EE; London, RE The Structural Basis for Nonsteroidal Anti-Inflammatory Drug Inhibition of Human Dihydrofolate Reductase. J Med Chem 63:8314-8324 (2020) [PubMed] Article More Info.:
Target
Name:
Bifunctional purine biosynthesis protein ATIC
Synonyms:
5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase | 5-aminoimidazole-4-carboxamide-ribonucleotide transformylase | AICAR Tfase | AICAR transformylase | ATIC | Aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) | Bifunctional purine biosynthesis protein PURH | IMP Cyclohydrolase (IMPCH) | IMP cyclohydrolase | IMP synthetase | Inosinicase | PUR9_HUMAN | PURH | Phosphoribosylaminoimidazolecarboxamide formyltransferase | Thymidylate synthase/GAR transformylase/AICAR transformylase
Type:
Protein
Mol. Mass.:
64616.62
Organism:
Homo sapiens (Human)
Description:
P31939
Residue:
592
Sequence:
MAPGQLALFSVSDKTGLVEFARNLTALGLNLVASGGTAKALRDAGLAVRDVSELTGFPEMLGGRVKTLHPAVHAGILARNIPEDNADMARLDFNLIRVVACNLYPFVKTVASPGVTVEEAVEQIDIGGVTLLRAAAKNHARVTVVCEPEDYVVVSTEMQSSESKDTSLETRRQLALKAFTHTAQYDEAISDYFRKQYSKGVSQMPLRYGMNPHQTPAQLYTLQPKLPITVLNGAPGFINLCDALNAWQLVKELKEALGIPAAASFKHVSPAGAAVGIPLSEDEAKVCMVYDLYKTLTPISAAYARARGADRMSSFGDFVALSDVCDVPTAKIISREVSDGIIAPGYEEEALTILSKKKNGNYCVLQMDQSYKPDENEVRTLFGLHLSQKRNNGVVDKSLFSNVVTKNKDLPESALRDLIVATIAVKYTQSNSVCYAKNGQVIGIGAGQQSRIHCTRLAGDKANYWWLRHHPQVLSMKFKTGVKRAEISNAIDQYVTGTIGEDEDLIKWKALFEEVPELLTEAEKKEWVEKLTEVSISSDAFFPFRDNVDRAKRSGVAYIAAPSGSAADKVVIEACDELGIILAHTNLRLFHH
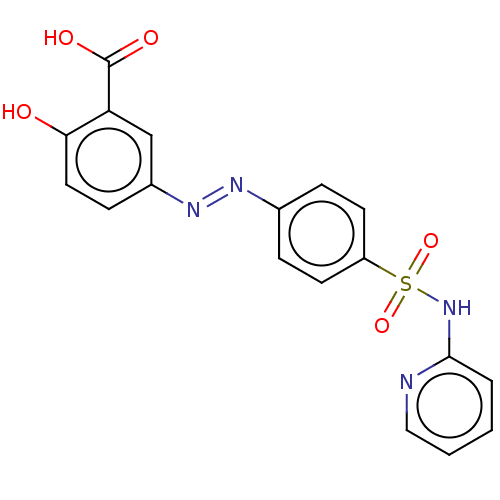
Inhibitor
Name:
BDBM50103596
Synonyms:
(E)-2-hydroxy-5-((4-(N-pyridin-2-ylsulfamoyl)-phenyl)diazenyl) benzoic acid (A8) | Azulfidine | Azulfidine EN | Azulfidine EN-Tabs | CHEBI:9334 | S.A.S. | S.A.S.-500 | SAS-500 | Salazopyrin | Salazosulfapyridine | Salicylazosulfapyridine | Sulfasalazine | Sulphasalazine | US11744839, Compound of formula 1
Type:
Small organic molecule
Emp. Form.:
C18H14N4O5S
Mol. Mass.:
398.393
SMILES:
OC(=O)c1cc(ccc1O)\N=N\c1ccc(cc1)S(=O)(=O)Nc1ccccn1