Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Envelope glycoprotein gp160
Ligand
BDBM50544715
Substrate
n/a
Meas. Tech.
ChEMBL_1993669 (CHEMBL4627564)
IC50
39±n/a nM
Citation
More Info.:
Target
Name:
Envelope glycoprotein gp160
Synonyms:
envelope glycoprotein
Type:
Enzyme Catalytic Domain
Mol. Mass.:
91798.58
Organism:
Human immunodeficiency virus 1
Description:
gi_45357394
Residue:
814
Sequence:
MRVKGIRRNYQHLWRWGTMLLGMLMICSAKEQLWVTAYYGVPVWKEATTTLFCASDAKAYDTEVHNVWATHACVPTDPNPREVVMGNVTEEFNIWNNSMVEQMHEDIISLWDESLKPCVKLTPLCVTFNCTNYNGTRNGTTTEPPEVKNCTTKETGIKNCSFNIATSGVEDRFKKEYALLYTADIVQIDNSSINYTLIGCNTSVITQACPKVSFEPIPIHYCAPAGFAILKCNNKTFNGKGPCTNVSTVQCTHGIRPVVSTQLLLNGSLAEEVVIRSDNFSDNAKTIIVQLKDPVVINCTRPNNNTRKGIRIGPGRTFYTTERIIGDIRQAHCNISRTQWNNTLRLIAAKLKKQFNNKTIIFRNSSGGDPEIVMHSFNCGGEFFYCNTTQLFNSTWVHNNTWVHNNTGNDTEEGTITLPCRIKQIINMWQEVGKAMYAPPIKGQIRCSSNITGLILTRDGGNTSSNNETFRPGGGDMRDNWRSELYKYKVVKIEPLGVAPTKARRRVVQREKRAVGMLGAMFLGFLGAAGSTMGAASLALTVQARQVVSGIVQQQNNLLRAIEAQQHLLQLTVWGIKQLQARVLAVERYLGDQQLLGIWGCSGKLICTTAVPWNDSWSNKSLKYIWDNMTWMQWEKEIDIHVDTIYQLLEASQYQQEKNEKDLLELDKWESLWNWFDITNWLWYIKIFIMIVGGLIGLRIVFTVLSIVNRVRQGYSPLSLQTRLPTQRGPDRPEGIEEEGGERQRHIRSISEWILNNYPGRPAEPVPLQLPPLERLTLDCNEDCGTSGTQGVGSPQVFVESPPVLESGTKEECC
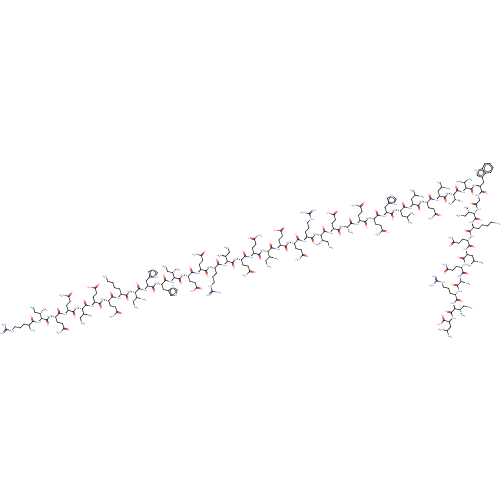
Inhibitor
Name:
BDBM50544715
Synonyms:
CHEMBL4647052
Type:
Small organic molecule
Emp. Form.:
C246H412N78O67
Mol. Mass.:
5534.3859
SMILES:
CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O |r|