Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysine-specific histone demethylase 1A
Ligand
BDBM424912
Substrate
n/a
Meas. Tech.
ChEMBL_1996292 (CHEMBL4630187)
Ki
6.1±n/a nM
Citation
 Gehling, VS; McGrath, JP; Duplessis, M; Khanna, A; Brucelle, F; Vaswani, RG; Côté, A; Stuckey, J; Watson, V; Cummings, RT; Balasubramanian, S; Iyer, P; Sawant, P; Good, AC; Albrecht, BK; Harmange, JC; Audia, JE; Bellon, SF; Trojer, P; Levell, JR Design and Synthesis of Styrenylcyclopropylamine LSD1 Inhibitors. ACS Med Chem Lett 11:1213-1220 (2020) [PubMed] Article
Gehling, VS; McGrath, JP; Duplessis, M; Khanna, A; Brucelle, F; Vaswani, RG; Côté, A; Stuckey, J; Watson, V; Cummings, RT; Balasubramanian, S; Iyer, P; Sawant, P; Good, AC; Albrecht, BK; Harmange, JC; Audia, JE; Bellon, SF; Trojer, P; Levell, JR Design and Synthesis of Styrenylcyclopropylamine LSD1 Inhibitors. ACS Med Chem Lett 11:1213-1220 (2020) [PubMed] Article More Info.:
Target
Name:
Lysine-specific histone demethylase 1A
Synonyms:
AOF2 | BRAF35-HDAC complex protein BHC110 | Flavin-containing amine oxidase domain-containing protein 2 | KDM1 | KDM1A | KDM1A_HUMAN | KIAA0601 | LSD1 | Lysine-specific demethylase 1 (LSD1) | Lysine-specific histone demethylase 1 | Lysine-specific histone demethylase 1 (LSD1)
Type:
Enzyme
Mol. Mass.:
92901.01
Organism:
Homo sapiens (Human)
Description:
O60341
Residue:
852
Sequence:
MLSGKKAAAAAAAAAAAATGTEAGPGTAGGSENGSEVAAQPAGLSGPAEVGPGAVGERTPRKKEPPRASPPGGLAEPPGSAGPQAGPTVVPGSATPMETGIAETPEGRRTSRRKRAKVEYREMDESLANLSEDEYYSEEERNAKAEKEKKLPPPPPQAPPEEENESEPEEPSGVEGAAFQSRLPHDRMTSQEAACFPDIISGPQQTQKVFLFIRNRTLQLWLDNPKIQLTFEATLQQLEAPYNSDTVLVHRVHSYLERHGLINFGIYKRIKPLPTKKTGKVIIIGSGVSGLAAARQLQSFGMDVTLLEARDRVGGRVATFRKGNYVADLGAMVVTGLGGNPMAVVSKQVNMELAKIKQKCPLYEANGQAVPKEKDEMVEQEFNRLLEATSYLSHQLDFNVLNNKPVSLGQALEVVIQLQEKHVKDEQIEHWKKIVKTQEELKELLNKMVNLKEKIKELHQQYKEASEVKPPRDITAEFLVKSKHRDLTALCKEYDELAETQGKLEEKLQELEANPPSDVYLSSRDRQILDWHFANLEFANATPLSTLSLKHWDQDDDFEFTGSHLTVRNGYSCVPVALAEGLDIKLNTAVRQVRYTASGCEVIAVNTRSTSQTFIYKCDAVLCTLPLGVLKQQPPAVQFVPPLPEWKTSAVQRMGFGNLNKVVLCFDRVFWDPSVNLFGHVGSTTASRGELFLFWNLYKAPILLALVAGEAAGIMENISDDVIVGRCLAILKGIFGSSAVPQPKETVVSRWRADPWARGSYSYVAAGSSGNDYDLMAQPITPGPSIPGAPQPIPRLFFAGEHTIRNYPATVHGALLSGLREAGRIADQFLGAMYTLPRQATPGVPAQQSPSM
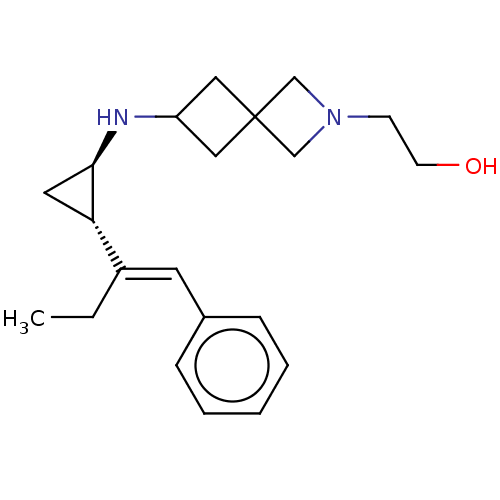
Inhibitor
Name:
BDBM424912
Synonyms:
2-(6-(((1R,2S)-2-((E)-1-phenylbut-1-en-2-yl)cyclopropyl)amino)-2-azaspiro[3.3]heptan-2-yl)ethanol | US10517849, Compound 1 | US11013718, Compound 1 | US11547695, Compound 1
Type:
Small organic molecule
Emp. Form.:
C21H30N2O
Mol. Mass.:
326.4757
SMILES:
CC\C(=C/c1ccccc1)[C@@H]1C[C@H]1NC1CC2(C1)CN(CCO)C2 |r|