Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM50169047
Substrate
n/a
Meas. Tech.
ChEMBL_2054175 (CHEMBL4709176)
IC50
2460±n/a nM
Citation
 Sisa, M; Dvorakova, M; Temml, V; Jarosova, V; Vanek, T; Landa, P Synthesis, inhibitory activity and in silico docking of dual COX/5-LOX inhibitors with quinone and resorcinol core. Eur J Med Chem 204:0 (2020) [PubMed] Article
Sisa, M; Dvorakova, M; Temml, V; Jarosova, V; Vanek, T; Landa, P Synthesis, inhibitory activity and in silico docking of dual COX/5-LOX inhibitors with quinone and resorcinol core. Eur J Med Chem 204:0 (2020) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
COX2 | Cyclooxygenase | Cyclooxygenase 2 (COX-2) | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2 AA) | Cyclooxygenase-2 (COX-2 AEA) | Cyclooxygenase-2 (COX-2) | PGH synthase 2 | PGH2_HUMAN | PGHS-2 | PHS II | PTGS2 | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2
Type:
Enzyme
Mol. Mass.:
69003.89
Organism:
Homo sapiens (Human)
Description:
Recombinant Cox-2 provided by Cayman (Cayman Chemical Co.,Ann Arbor, MI).
Residue:
604
Sequence:
MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFLTRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADYGYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKYQIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCDVLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQNRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRVAGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEALYGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEVGFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKERSTEL
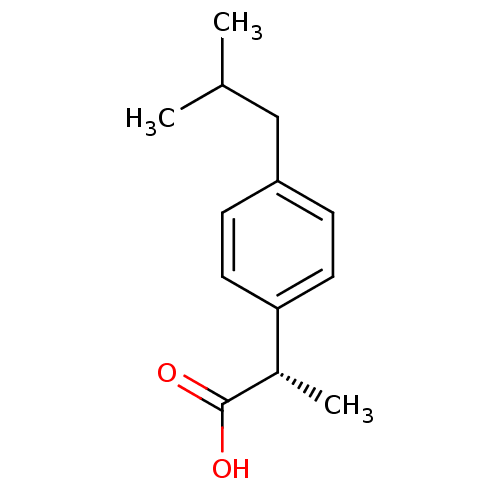
Inhibitor
Name:
BDBM50169047
Synonyms:
(S)-2-(4-Isobutyl-phenyl)-propionic acid | (S)-2-(4-isobutylphenyl)propanoic acid | (S)-ibuprofen | CHEMBL175 | DEXIBUPROFEN | IBUPROFEN LYSINE
Type:
Small organic molecule
Emp. Form.:
C13H18O2
Mol. Mass.:
206.2808
SMILES:
CC(C)Cc1ccc(cc1)[C@H](C)C(O)=O |r|