Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
Ligand
BDBM50179360
Substrate
n/a
Meas. Tech.
ChEMBL_2055183 (CHEMBL4710184)
IC50
890±n/a nM
Citation
 Ahmad, H; Ullah, S; Rahman, F; Saeed, A; Pelletier, J; Sévigny, J; Hassan, A; Iqbal, J Synthesis of biphenyl oxazole derivatives via Suzuki coupling and biological evaluations as nucleotide pyrophosphatase/phosphodiesterase-1 and -3 inhibitors. Eur J Med Chem 208:0 (2020) [PubMed] Article
Ahmad, H; Ullah, S; Rahman, F; Saeed, A; Pelletier, J; Sévigny, J; Hassan, A; Iqbal, J Synthesis of biphenyl oxazole derivatives via Suzuki coupling and biological evaluations as nucleotide pyrophosphatase/phosphodiesterase-1 and -3 inhibitors. Eur J Med Chem 208:0 (2020) [PubMed] Article More Info.:
Target
Name:
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
Synonyms:
Alkaline phosphodiesterase I | CD_antigen=CD203c | E-NPP 3 | ENPP3 | ENPP3_HUMAN | Ectonucleotide pyrophosphatase/phosphodiesterase family member 3 | NPPase | Nucleotide pyrophosphatase | PD-Ibeta | PDNP3 | Phosphodiesterase I beta | Phosphodiesterase I/nucleotide pyrophosphatase 3
Type:
PROTEIN
Mol. Mass.:
100124.29
Organism:
Homo sapiens (Human)
Description:
ChEMBL_101240
Residue:
875
Sequence:
MESTLTLATEQPVKKNTLKKYKIACIVLLALLVIMSLGLGLGLGLRKLEKQGSCRKKCFDASFRGLENCRCDVACKDRGDCCWDFEDTCVESTRIWMCNKFRCGETRLEASLCSCSDDCLQRKDCCADYKSVCQGETSWLEENCDTAQQSQCPEGFDLPPVILFSMDGFRAEYLYTWDTLMPNINKLKTCGIHSKYMRAMYPTKTFPNHYTIVTGLYPESHGIIDNNMYDVNLNKNFSLSSKEQNNPAWWHGQPMWLTAMYQGLKAATYFWPGSEVAINGSFPSIYMPYNGSVPFEERISTLLKWLDLPKAERPRFYTMYFEEPDSSGHAGGPVSARVIKALQVVDHAFGMLMEGLKQRNLHNCVNIILLADHGMDQTYCNKMEYMTDYFPRINFFYMYEGPAPRIRAHNIPHDFFSFNSEEIVRNLSCRKPDQHFKPYLTPDLPKRLHYAKNVRIDKVHLFVDQQWLAVRSKSNTNCGGGNHGYNNEFRSMEAIFLAHGPSFKEKTEVEPFENIEVYNLMCDLLRIQPAPNNGTHGSLNHLLKVPFYEPSHAEEVSKFSVCGFANPLPTESLDCFCPHLQNSTQLEQVNQMLNLTQEEITATVKVNLPFGRPRVLQKNVDHCLLYHREYVSGFGKAMRMPMWSSYTVPQLGDTSPLPPTVPDCLRADVRVPPSESQKCSFYLADKNITHGFLYPPASNRTSDSQYDALITSNLVPMYEEFRKMWDYFHSVLLIKHATERNGVNVVSGPIFDYNYDGHFDAPDEITKHLANTDVPIPTHYFVVLTSCKNKSHTPENCPGWLDVLPFIIPHRPTNVESCPEGKPEALWVEERFTAHIARVRDVELLTGLDFYQDKVQPVSEILQLKTYLPTFETTI
Inhibitor
Name:
BDBM50179360
Synonyms:
CHEMBL3040216
Type:
Small organic molecule
Emp. Form.:
C51H40N6O23S6
Mol. Mass.:
1297.28
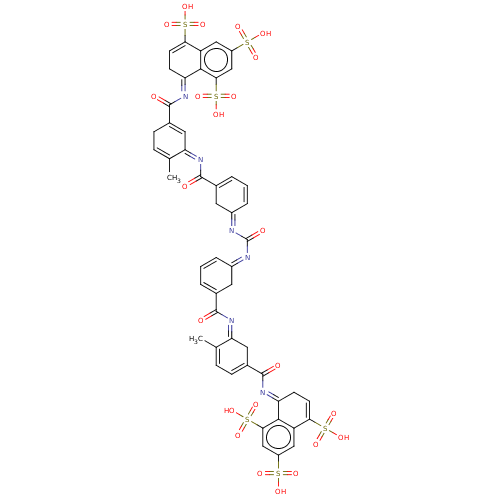
SMILES:
CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11|