Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nucleosome-remodeling factor subunit BPTF
Ligand
BDBM25121
Substrate
n/a
Meas. Tech.
ChEMBL_2120819 (CHEMBL4829966)
Kd
37000±n/a nM
Citation
More Info.:
Target
Name:
Nucleosome-remodeling factor subunit BPTF
Synonyms:
BPTF | BPTF_HUMAN | Bromodomain and PHD finger-containing transcription factor | FAC1 | FALZ | Fetal Alz-50 clone 1 protein | Fetal Alzheimer antigen
Type:
PROTEIN
Mol. Mass.:
338257.07
Organism:
Homo sapiens (Human)
Description:
ChEMBL_107969
Residue:
3046
Sequence:
MRGRRGRPPKQPAAPAAERCAPAPPPPPPPPTSGPIGGLRSRHRGSSRGRWAAAQAEVAPKTRLSSPRGGSSSRRKPPPPPPAPPSTSAPGRGGRGGGGGRTGGGGGGGHLARTTAARRAVNKVVYDDHESEEEEEEEDMVSEEEEEEDGDAEETQDSEDDEEDEMEEDDDDSDYPEEMEDDDDDASYCTESSFRSHSTYSSTPGRRKPRVHRPRSPILEEKDIPPLEFPKSSEDLMVPNEHIMNVIAIYEVLRNFGTVLRLSPFRFEDFCAALVSQEQCTLMAEMHVVLLKAVLREEDTSNTTFGPADLKDSVNSTLYFIDGMTWPEVLRVYCESDKEYHHVLPYQEAEDYPYGPVENKIKVLQFLVDQFLTTNIAREELMSEGVIQYDDHCRVCHKLGDLLCCETCSAVYHLECVKPPLEEVPEDEWQCEVCVAHKVPGVTDCVAEIQKNKPYIRHEPIGYDRSRRKYWFLNRRLIIEEDTENENEKKIWYYSTKVQLAELIDCLDKDYWEAELCKILEEMREEIHRHMDITEDLTNKARGSNKSFLAAANEEILESIRAKKGDIDNVKSPEETEKDKNETENDSKDAEKNREEFEDQSLEKDSDDKTPDDDPEQGKSEEPTEVGDKGNSVSANLGDNTTNATSEETSPSEGRSPVGCLSETPDSSNMAEKKVASELPQDVPEEPNKTCESSNTSATTTSIQPNLENSNSSSELNSSQSESAKAADDPENGERESHTPVSIQEEIVGDFKSEKSNGELSESPGAGKGASGSTRIITRLRNPDSKLSQLKSQQVAAAAHEANKLFKEGKEVLVVNSQGEISRLSTKKEVIMKGNINNYFKLGQEGKYRVYHNQYSTNSFALNKHQHREDHDKRRHLAHKFCLTPAGEFKWNGSVHGSKVLTISTLRLTITQLENNIPSSFLHPNWASHRANWIKAVQMCSKPREFALALAILECAVKPVVMLPIWRESLGHTRLHRMTSIEREEKEKVKKKEKKQEEEETMQQATWVKYTFPVKHQVWKQKGEEYRVTGYGGWSWISKTHVYRFVPKLPGNTNVNYRKSLEGTKNNMDENMDESDKRKCSRSPKKIKIEPDSEKDEVKGSDAAKGADQNEMDISKITEKKDQDVKELLDSDSDKPCKEEPMEVDDDMKTESHVNCQESSQVDVVNVSEGFHLRTSYKKKTKSSKLDGLLERRIKQFTLEEKQRLEKIKLEGGIKGIGKTSTNSSKNLSESPVITKAKEGCQSDSMRQEQSPNANNDQPEDLIQGCSESDSSVLRMSDPSHTTNKLYPKDRVLDDVSIRSPETKCPKQNSIENDIEEKVSDLASRGQEPSKSKTKGNDFFIDDSKLASADDIGTLICKNKKPLIQEESDTIVSSSKSALHSSVPKSTNDRDATPLSRAMDFEGKLGCDSESNSTLENSSDTVSIQDSSEEDMIVQNSNESISEQFRTREQDVEVLEPLKCELVSGESTGNCEDRLPVKGTEANGKKPSQQKKLEERPVNKCSDQIKLKNTTDKKNNENRESEKKGQRTSTFQINGKDNKPKIYLKGECLKEISESRVVSGNVEPKVNNINKIIPENDIKSLTVKESAIRPFINGDVIMEDFNERNSSETKSHLLSSSDAEGNYRDSLETLPSTKESDSTQTTTPSASCPESNSVNQVEDMEIETSEVKKVTSSPITSEEESNLSNDFIDENGLPINKNENVNGESKRKTVITEVTTMTSTVATESKTVIKVEKGDKQTVVSSTENCAKSTVTTTTTTVTKLSTPSTGGSVDIISVKEQSKTVVTTTVTDSLTTTGGTLVTSMTVSKEYSTRDKVKLMKFSRPKKTRSGTALPSYRKFVTKSSKKSIFVLPNDDLKKLARKGGIREVPYFNYNAKPALDIWPYPSPRPTFGITWRYRLQTVKSLAGVSLMLRLLWASLRWDDMAAKAPPGGGTTRTETSETEITTTEIIKRRDVGPYGIRSEYCIRKIICPIGVPETPKETPTPQRKGLRSSALRPKRPETPKQTGPVIIETWVAEEELELWEIRAFAERVEKEKAQAVEQQAKKRLEQQKPTVIATSTTSPTSSTTSTISPAQKVMVAPISGSVTTGTKMVLTTKVGSPATVTFQQNKNFHQTFATWVKQGQSNSGVVQVQQKVLGIIPSSTGTSQQTFTSFQPRTATVTIRPNTSGSGGTTSNSQVITGPQIRPGMTVIRTPLQQSTLGKAIIRTPVMVQPGAPQQVMTQIIRGQPVSTAVSAPNTVSSTPGQKSLTSATSTSNIQSSASQPPRPQQGQVKLTMAQLTQLTQGHGGNQGLTVVIQGQGQTTGQLQLIPQGVTVLPGPGQQLMQAAMPNGTVQRFLFTPLATTATTASTTTTTVSTTAAGTGEQRQSKLSPQMQVHQDKTLPPAQSSSVGPAEAQPQTAQPSAQPQPQTQPQSPAQPEVQTQPEVQTQTTVSSHVPSEAQPTHAQSSKPQVAAQSQPQSNVQGQSPVRVQSPSQTRIRPSTPSQLSPGQQSQVQTTTSQPIPIQPHTSLQIPSQGQPQSQPQVQSSTQTLSSGQTLNQVTVSSPSRPQLQIQQPQPQVIAVPQLQQQVQVLSQIQSQVVAQIQAQQSGVPQQIKLQLPIQIQQSSAVQTHQIQNVVTVQAASVQEQLQRVQQLRDQQQKKKQQQIEIKREHTLQASNQSEIIQKQVVMKHNAVIEHLKQKKSMTPAEREENQRMIVCNQVMKYILDKIDKEEKQAAKKRKREESVEQKRSKQNATKLSALLFKHKEQLRAEILKKRALLDKDLQIEVQEELKRDLKIKKEKDLMQLAQATAVAAPCPPVTPAPPAPPAPPPSPPPPPAVQHTGLLSTPTLPAASQKRKREEEKDSSSKSKKKKMISTTSKETKKDTKLYCICKTPYDESKFYIGCDRCQNWYHGRCVGILQSEAELIDEYVCPQCQSTEDAMTVLTPLTEKDYEGLKRVLRSLQAHKMAWPFLEPVDPNDAPDYYGVIKEPMDLATMEERVQRRYYEKLTEFVADMTKIFDNCRYYNPSDSPFYQCAEVLESFFVQKLKGFKASRSHNNKLQSTAS
Inhibitor
Name:
BDBM25121
Synonyms:
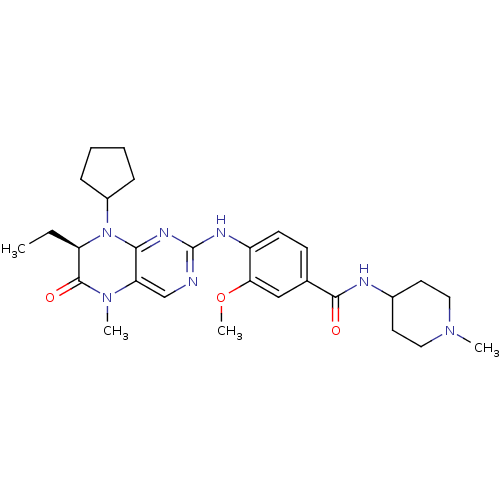
4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl]amino}-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide | BI 2536 | CHEMBL513909 | US10450297, Example 300 | US8598172, 5
Type:
Small organic molecule
Emp. Form.:
C28H39N7O3
Mol. Mass.:
521.6544
SMILES:
CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r|