Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Protein mono-ADP-ribosyltransferase PARP4
Ligand
BDBM50541697
Substrate
n/a
Meas. Tech.
ChEMBL_2146114 (CHEMBL5030394)
IC50
>10000±n/a nM
Citation
 Leenders, RGG; Brinch, SA; Sowa, ST; Amundsen-Isaksen, E; Galera-Prat, A; Murthy, S; Aertssen, S; Smits, JN; Nieczypor, P; Damen, E; Wegert, A; Nazaré, M; Lehtiö, L; Waaler, J; Krauss, S Development of a 1,2,4-Triazole-Based Lead Tankyrase Inhibitor: Part II. J Med Chem 64:17936-17949 (2021) [PubMed] Article
Leenders, RGG; Brinch, SA; Sowa, ST; Amundsen-Isaksen, E; Galera-Prat, A; Murthy, S; Aertssen, S; Smits, JN; Nieczypor, P; Damen, E; Wegert, A; Nazaré, M; Lehtiö, L; Waaler, J; Krauss, S Development of a 1,2,4-Triazole-Based Lead Tankyrase Inhibitor: Part II. J Med Chem 64:17936-17949 (2021) [PubMed] Article More Info.:
Target
Name:
Protein mono-ADP-ribosyltransferase PARP4
Synonyms:
(ARTD4 or PARP4) | 193 kDa vault protein | 2.4.2.- | 2.4.2.30 | ADP-ribosyltransferase diphtheria toxin-like 4 | ADPRTL1 | ADPRTL1 | ARTD4 | KIAA0177 | KIAA0177 GN | PARP-4 | PARP-related/IalphaI-related H5/proline-rich | PARP4 | PARP4_HUMAN | PARPL | PH5P | Poly [ADP-ribose] polymerase 4 | Synonyms=ADPRTL1 | VPARP | Vault poly(ADP-ribose) polymerase
Type:
n/a
Mol. Mass.:
192563.23
Organism:
Homo sapiens (Human)
Description:
Q9UKK3
Residue:
1724
Sequence:
MVMGIFANCIFCLKVKYLPQQQKKKLQTDIKENGGKFSFSLNPQCTHIILDNADVLSQYQLNSIQKNHVHIANPDFIWKSIREKRLLDVKNYDPYKPLDITPPPDQKASSSEVKTEGLCPDSATEEEDTVELTEFGMQNVEIPHLPQDFEVAKYNTLEKVGMEGGQEAVVVELQCSRDSRDCPFLISSHFLLDDGMETRRQFAIKKTSEDASEYFENYIEELKKQGFLLREHFTPEATQLASEQLQALLLEEVMNSSTLSQEVSDLVEMIWAEALGHLEHMLLKPVNRISLNDVSKAEGILLLVKAALKNGETAEQLQKMMTEFYRLIPHKGTMPKEVNLGLLAKKADLCQLIRDMVNVCETNLSKPNPPSLAKYRALRCKIEHVEQNTEEFLRVRKEVLQNHHSKSPVDVLQIFRVGRVNETTEFLSKLGNVRPLLHGSPVQNIVGILCRGLLLPKVVEDRGVQRTDVGNLGSGIYFSDSLSTSIKYSHPGETDGTRLLLICDVALGKCMDLHEKDFSLTEAPPGYDSVHGVSQTASVTTDFEDDEFVVYKTNQVKMKYIIKFSMPGDQIKDFHPSDHTELEEYRPEFSNFSKVEDYQLPDAKTSSSTKAGLQDASGNLVPLEDVHIKGRIIDTVAQVIVFQTYTNKSHVPIEAKYIFPLDDKAAVCGFEAFINGKHIVGEIKEKEEAQQEYLEAVTQGHGAYLMSQDAPDVFTVSVGNLPPKAKVLIKITYITELSILGTVGVFFMPATVAPWQQDKALNENLQDTVEKICIKEIGTKQSFSLTMSIEMPYVIEFIFSDTHELKQKRTDCKAVISTMEGSSLDSSGFSLHIGLSAAYLPRMWVEKHPEKESEACMLVFQPDLDVDLPDLASESEVIICLDCSSSMEGVTFLQAKQIALHALSLVGEKQKVNIIQFGTGYKELFSYPKHITSNTMAAEFIMSATPTMGNTDFWKTLRYLSLLYPARGSRNILLVSDGHLQDESLTLQLVKRSRPHTRLFACGIGSTANRHVLRILSQCGAGVFEYFNAKSKHSWRKQIEDQMTRLCSPSCHSVSVKWQQLNPDVPEALQAPAQVPSLFLNDRLLVYGFIPHCTQATLCALIQEKEFRTMVSTTELQKTTGTMIHKLAARALIRDYEDGILHENETSHEMKKQTLKSLIIKLSKENSLITQFTSFVAVEKRDENESPFPDIPKVSELIAKEDVDFLPYMSWQGEPQEAVRNQSLLASSEWPELRLSKRKHRKIPFSKRKMELSQPEVSEDFEEDGLGVLPAFTSNLERGGVEKLLDLSWTESCKPTATEPLFKKVSPWETSTSSFFPILAPAVGSYLPPTARAHSPASLSFASYRQVASFGSAAPPRQFDASQFSQGPVPGTCADWIPQSASCPTGPPQNPPSSPYCGIVFSGSSLSSAQSAPLQHPGGFTTRPSAGTFPELDSPQLHFSLPTDPDPIRGFGSYHPSASSPFHFQPSAASLTANLRLPMASALPEALCSQSRTTPVDLCLLEESVGSLEGSRCPVFAFQSSDTESDELSEVLQDSCFLQIKCDTKDDSILCFLEVKEEDEIVCIQHWQDAVPWTELLSLQTEDGFWKLTPELGLILNLNTNGLHSFLKQKGIQSLGVKGRECLLDLIATMLVLQFIRTRLEKEGIVFKSLMKMDDASISRNIPWAFEAIKQASEWVRRTEGQYPSICPRLELGNDWDSATKQLLGLQPISTVSPLHRVLHYSQG
Inhibitor
Name:
BDBM50541697
Synonyms:
CHEMBL4633637
Type:
Small organic molecule
Emp. Form.:
C25H23FN6O2
Mol. Mass.:
458.4875
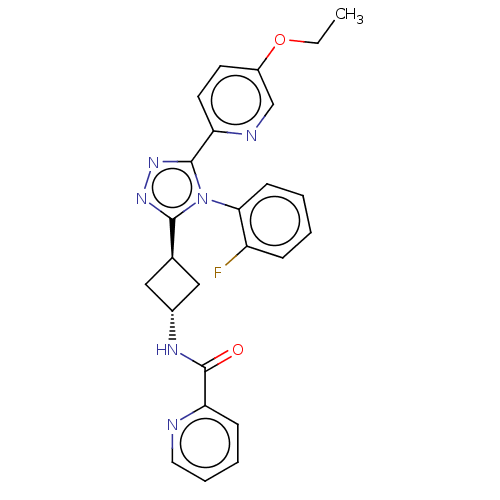
SMILES:
CCOc1ccc(nc1)-c1nnc([C@H]2C[C@@H](C2)NC(=O)c2ccccn2)n1-c1ccccc1F |r,wU:15.18,wD:13.13,(2.31,-18.95,;3.77,-18.47,;4.92,-19.5,;6.38,-19.02,;7.54,-20.05,;9,-19.57,;9.31,-18.07,;8.17,-17.04,;6.7,-17.51,;10.77,-17.6,;11.25,-16.13,;12.79,-16.13,;13.26,-17.6,;14.73,-18.07,;15.42,-19.45,;16.8,-18.75,;16.1,-17.38,;18.26,-19.23,;19.41,-18.2,;19.09,-16.69,;20.87,-18.67,;22,-17.64,;23.47,-18.11,;23.79,-19.62,;22.65,-20.65,;21.19,-20.18,;12.02,-18.5,;12.02,-20.04,;10.68,-20.81,;10.68,-22.34,;12.02,-23.12,;13.36,-22.34,;13.35,-20.8,;14.68,-20.03,)|