Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
cAMP-dependent protein kinase catalytic subunit alpha
Ligand
BDBM27248
Substrate
n/a
Meas. Tech.
ChEMBL_420145 (CHEMBL912715)
IC50
8.30±n/a nM
Citation
 Enkvist, E; Lavogina, D; Raidaru, G; Vaasa, A; Viil, I; Lust, M; Viht, K; Uri, A Conjugation of adenosine and hexa-(D-arginine) leads to a nanomolar bisubstrate-analog inhibitor of basophilic protein kinases. J Med Chem 49:7150-9 (2006) [PubMed] Article
Enkvist, E; Lavogina, D; Raidaru, G; Vaasa, A; Viil, I; Lust, M; Viht, K; Uri, A Conjugation of adenosine and hexa-(D-arginine) leads to a nanomolar bisubstrate-analog inhibitor of basophilic protein kinases. J Med Chem 49:7150-9 (2006) [PubMed] Article More Info.:
Target
Name:
cAMP-dependent protein kinase catalytic subunit alpha
Synonyms:
KAPCA_HUMAN | PKA C-alpha | PKACA | PRKACA | cAMP-dependent protein kinase (PKA) | cAMP-dependent protein kinase catalytic (PKA) | cAMP-dependent protein kinase catalytic subunit alpha | cAMP-dependent protein kinase catalytic subunit alpha (PKA) | cAMP-dependent protein kinase catalytic subunit alpha (PKACA) | cAMP-dependent protein kinase catalytic subunit alpha (PKAc) | cAMP-dependent protein kinase catalytic subunit alpha isoform 1 | cAMP-dependent protein kinase, alpha-catalytic subunit
Type:
Enzyme Catalytic Subunit
Mol. Mass.:
40598.73
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
351
Sequence:
MGNAAAAKKGSEQESVKEFLAKAKEDFLKKWESPAQNTAHLDQFERIKTLGTGSFGRVMLVKHKETGNHYAMKILDKQKVVKLKQIEHTLNEKRILQAVNFPFLVKLEFSFKDNSNLYMVMEYVPGGEMFSHLRRIGRFSEPHARFYAAQIVLTFEYLHSLDLIYRDLKPENLLIDQQGYIQVTDFGFAKRVKGRTWTLCGTPEYLAPEIILSKGYNKAVDWWALGVLIYEMAAGYPPFFADQPIQIYEKIVSGKVRFPSHFSSDLKDLLRNLLQVDLTKRFGNLKNGVNDIKNHKWFATTDWIAIYQRKVEAPFIPKFKGPGDTSNFDDYEEEEIRVSINEKCGKEFSEF
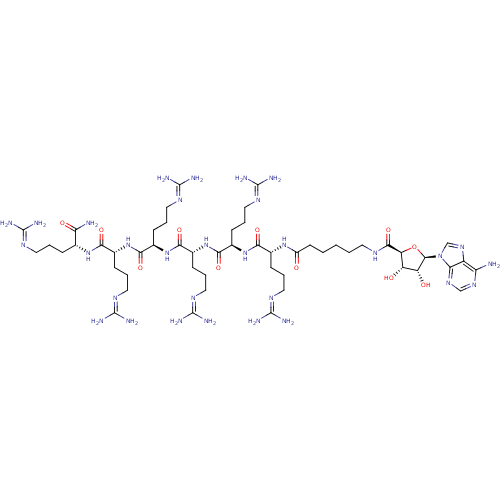
Inhibitor
Name:
BDBM27248
Synonyms:
6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]formamido}-N-[(1R)-4-carbamimidamido-1-{[(1R)-4-carbamimidamido-1-{[(1R)-4-carbamimidamido-1-{[(1R)-4-carbamimidamido-1-{[(1R)-4-carbamimidamido-1-{[(1R)-4-carbamimidamido-1-carbamoylbutyl]carbamoyl}butyl]carbamoyl}butyl]carbamoyl}butyl]carbamoyl}butyl]carbamoyl}butyl]hexanamide | ARC-902 | CHEMBL279185
Type:
Small organic molecule
Emp. Form.:
C52H95N31O11
Mol. Mass.:
1330.5118
SMILES:
[#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r|