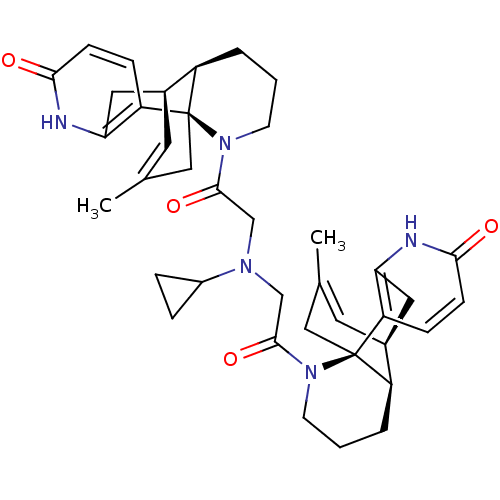
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acetylcholinesterase
Ligand
BDBM50199541
Substrate
n/a
Meas. Tech.
ChEMBL_441060 (CHEMBL890218)
pH
5±n/a
IC50
1002±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
Acetylcholinesterase
Synonyms:
ACES_RAT | Acetylcholinesterase (AChE) | Acetylcholinesterase and butyrylcholinesterase (AChE and BChE) | Acetylcholinesterase precursor | Acetylcholinesterase, AChE | Ache
Type:
Enzyme
Mol. Mass.:
68193.62
Organism:
Rattus norvegicus (rat)
Description:
P37136
Residue:
614
Sequence:
MRPPWYPLHTPSLASPLLFLLLSLLGGGARAEGREDPQLLVRVRGGQLRGIRLKAPGGPVSAFLGIPFAEPPVGSRRFMPPEPKRPWSGILDATTFQNVCYQYVDTLYPGFEGTEMWNPNRELSEDCLYLNVWTPYPRPTSPTPVLIWIYGGGFYSGASSLDVYDGRFLAQVEGTVLVSMNYRVGTFGFLALPGSREAPGNVGLLDQRLALQWVQENIAAFGGDPMSVTLFGESAGAASVGMHILSLPSRSLFHRAVLQSGTPNGPWATVSAGEARRRATLLARLVGCPPGGAGGNDTELISCLRTRPAQDLVDHEWHVLPQESIFRFSFVPVVDGDFLSDTPDALINTGDFQDLQVLVGVVKDEGSYFLVYGVPGFSKDNESLISRAQFLAGVRIGVPQASDLAAEAVVLHYTDWLHPEDPAHLRDAMSAVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLTWPLWMGVPHGYEIEFIFGLPLDPSLNYTVEERIFAQRLMQYWTNFARTGDPNDPRDSKSPRWPPYTTAAQQYVSLNLKPLEVRRGLRAQTCAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKNQFDHYSKQERCSDL
Inhibitor
Name:
BDBM50199541
Synonyms:
CHEMBL397501 | N,N-bis(1-oxo-8,15-didehydrolycodinocarbonylmethyl)-cyclopropylamine
Type:
Small organic molecule
Emp. Form.:
C39H47N5O4
Mol. Mass.:
649.8216
SMILES:
CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@@]3(C1)[C@@H]2CCCN3C(=O)CN(CC(=O)N1CCC[C@@H]2[C@@H]3Cc4[nH]c(=O)ccc4[C@]12CC(C)=C3)C1CC1 |c:50,t:1,TLB:10:11:14:13.2.1,6:5:14:13.2.1,THB:43:42:30:32.33.39|