Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Growth hormone secretagogue receptor type 1
Ligand
BDBM50260271
Substrate
n/a
Meas. Tech.
ChEMBL_509093 (CHEMBL1007237)
IC50
12±n/a nM
Citation
 Holst, B; Mokrosinski, J; Lang, M; Brandt, E; Nygaard, R; Frimurer, TM; Beck-Sickinger, AG; Schwartz, TW Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem 282:15799-811 (2007) [PubMed] Article
Holst, B; Mokrosinski, J; Lang, M; Brandt, E; Nygaard, R; Frimurer, TM; Beck-Sickinger, AG; Schwartz, TW Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem 282:15799-811 (2007) [PubMed] Article More Info.:
Target
Name:
Growth hormone secretagogue receptor type 1
Synonyms:
GH-releasing peptide receptor | GHRP | GHS-R | GHSR | GHSR_HUMAN | Ghrelin Receptor (Growth Hormone Secretagogue Receptor Type 1) | Ghrelin receptor | Ghrelin receptor 1a (GHS-R1a)
Type:
Receptor
Mol. Mass.:
41334.57
Organism:
Homo sapiens (Human)
Description:
Receptor binding studies use plasma membranes from LLC PK-1 cells transiently transfected with hGHSR1a.
Residue:
366
Sequence:
MWNATPSEEPGFNLTLADLDWDASPGNDSLGDELLQLFPAPLLAGVTATCVALFVVGIAGNLLTMLVVSRFRELRTTTNLYLSSMAFSDLLIFLCMPLDLVRLWQYRPWNFGDLLCKLFQFVSESCTYATVLTITALSVERYFAICFPLRAKVVVTKGRVKLVIFVIWAVAFCSAGPIFVLVGVEHENGTDPWDTNECRPTEFAVRSGLLTVMVWVSSIFFFLPVFCLTVLYSLIGRKLWRRRRGDAVVGASLRDQNHKQTVKMLAVVVFAFILCWLPFHVGRYLFSKSFEPGSLEIAQISQYCNLVSFVLFYLSAAINPILYNIMSKKYRVAVFRLLGFEPFSQRKLSTLKDESSRAWTESSINT
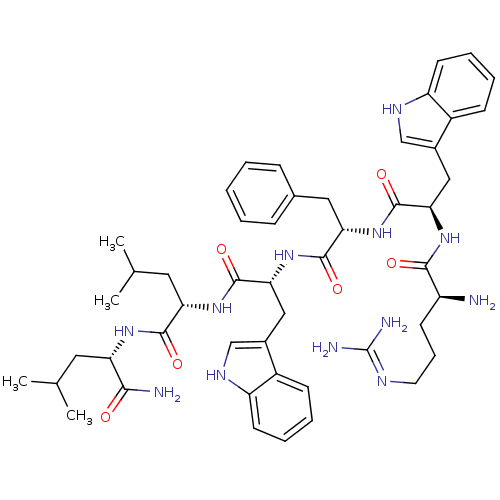
Inhibitor
Name:
BDBM50260271
Synonyms:
CHEMBL500703 | RwFwLL-NH2
Type:
Small organic molecule
Emp. Form.:
C49H66N12O6
Mol. Mass.:
919.1251
SMILES:
CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)CCCN=C(N)N)C(N)=O |r,wU:30.40,16.28,55.60,8.12,41.56,wD:4.4,(13.77,-48.69,;13.77,-47.15,;15.1,-46.38,;12.44,-46.38,;12.44,-44.84,;11.1,-44.08,;9.77,-44.85,;9.77,-46.39,;8.44,-44.08,;8.43,-42.54,;9.77,-41.77,;9.77,-40.23,;11.1,-42.54,;7.1,-44.85,;5.76,-44.09,;5.76,-42.55,;4.42,-44.86,;4.43,-46.4,;5.76,-47.16,;7.17,-46.53,;8.2,-47.68,;7.43,-49.01,;7.9,-50.48,;6.88,-51.62,;5.37,-51.3,;4.9,-49.84,;5.93,-48.69,;3.09,-44.08,;1.76,-44.85,;1.76,-46.39,;.43,-44.08,;.43,-42.55,;1.76,-41.77,;3.09,-42.54,;4.43,-41.77,;4.42,-40.23,;3.08,-39.47,;1.75,-40.24,;-.9,-44.85,;-2.24,-44.09,;-2.24,-42.56,;-3.57,-44.86,;-3.57,-46.4,;-2.24,-47.17,;-.84,-46.55,;.2,-47.69,;-.57,-49.02,;-.09,-50.48,;-1.13,-51.63,;-2.62,-51.31,;-3.1,-49.84,;-2.08,-48.7,;-4.9,-44.09,;-6.24,-44.86,;-6.24,-46.4,;-7.58,-44.09,;-8.89,-44.86,;-7.58,-42.55,;-6.24,-41.78,;-6.24,-40.24,;-4.91,-39.47,;-4.91,-37.93,;-6.23,-37.16,;-3.57,-37.15,;13.77,-44.07,;15.1,-44.85,;13.76,-42.53,)|