Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histamine H3 receptor
Ligand
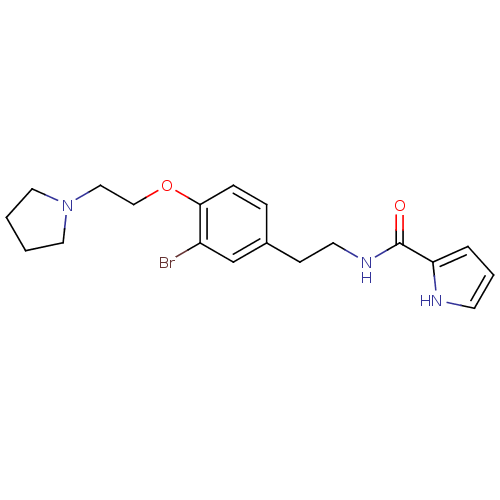
BDBM50293613
Substrate
n/a
Meas. Tech.
ChEMBL_571442 (CHEMBL1032753)
Ki
150±n/a nM
Citation
 Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett 19:3204-8 (2009) [PubMed] Article
Kennedy, JP; Conn, PJ; Lindsley, CW A novel class of H3 antagonists derived from the natural product guided synthesis of unnatural analogs of the marine bromopyrrole alkaloid dispyrin. Bioorg Med Chem Lett 19:3204-8 (2009) [PubMed] Article More Info.:
Target
Name:
Histamine H3 receptor
Synonyms:
G-protein coupled receptor 97 | GPCR97 | HH3R | HISTAMINE H3 | HRH3 | HRH3_HUMAN | Histamine H3 receptor (H3) | Histamine H3L | Histamine receptor (H3 and H4)
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
48691.47
Organism:
Homo sapiens (Human)
Description:
Binding assays were using CHO cells stably expressing hH3R receptors.
Residue:
445
Sequence:
MERAPPDGPLNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFVADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCTSSAFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMLLVWVLAFLLYGPAILSWEYLSGGSSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGAREAAGPEPPPEAQPSPPPPPGCWGCWQKGHGEAMPLHRYGVGEAAVGAEAGEATLGGGGGGGSVASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSFTQRFRLSRDRKVAKSLAVIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHHSFRRAFTKLLCPQKLKIQPHSSLEHCWK