Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM35509
Substrate
n/a
Meas. Tech.
ChEMBL_599107 (CHEMBL1047162)
IC50
110±n/a nM
Citation
 Sidique, S; Shiryaev, SA; Ratnikov, BI; Herath, A; Su, Y; Strongin, AY; Cosford, ND Structure-activity relationship and improved hydrolytic stability of pyrazole derivatives that are allosteric inhibitors of West Nile Virus NS2B-NS3 proteinase. Bioorg Med Chem Lett 19:5773-7 (2009) [PubMed] Article
Sidique, S; Shiryaev, SA; Ratnikov, BI; Herath, A; Su, Y; Strongin, AY; Cosford, ND Structure-activity relationship and improved hydrolytic stability of pyrazole derivatives that are allosteric inhibitors of West Nile Virus NS2B-NS3 proteinase. Bioorg Med Chem Lett 19:5773-7 (2009) [PubMed] Article More Info.:
Target
Name:
Genome polyprotein
Synonyms:
POLG_WNV | polyprotein precursor
Type:
PROTEIN
Mol. Mass.:
380170.63
Organism:
West Nile virus
Description:
ChEMBL_1438217
Residue:
3430
Sequence:
MSKKPGGPGKNRAVNMLKRGMPRGLSLIGLKRAMLSLIDGKGPIRFVLALLAFFRFTAIAPTRAVLDRWRGVNKQTAMKHLLSFKKELGTLTSAINRRSTKQKKRGGTAGFTILLGLIACAGAVTLSNFQGKVMMTVNATDVTDVITIPTAAGKNLCIVRAMDVGYLCEDTITYECPVLAAGNDPEDIDCWCTKSSVYVRYGRCTKTRHSRRSRRSLTVQTHGESTLANKKGAWLDSTKATRYLVKTESWILRNPGYALVAAVIGWMLGSNTMQRVVFAILLLLVAPAYSFNCLGMSNRDFLEGVSGATWVDLVLEGDSCVTIMSKDKPTIDVKMMNMEAANLADVRSYCYLASVSDLSTRAACPTMGEAHNEKRADPAFVCKQGVVDRGWGNGCGLFGKGSIDTCAKFACTTKATGWIIQKENIKYEVAIFVHGPTTVESHGKIGATQAGRFSITPSAPSYTLKLGEYGEVTVDCEPRSGIDTSAYYVMSVGEKSFLVHREWFMDLNLPWSSAGSTTWRNRETLMEFEEPHATKQSVVALGSQEGALHQALAGAIPVEFSSNTVKLTSGHLKCRVKMEKLQLKGTTYGVCSKAFKFARTPADTGHGTVVLELQYTGTDGPCKVPISSVASLNDLTPVGRLVTVNPFVSVATANSKVLIELEPPFGDSYIVVGRGEQQINHHWHKSGSSIGKAFTTTLRGAQRLAALGDTAWDFGSVGGVFTSVGKAIHQVFGGAFRSLFGGMSWITQGLLGALLLWMGINARDRSIAMTFLAVGGVLLFLSVNVHADTGCAIDIGRQELRCGSGVFIHNDVEAWMDRYKFYPETPQGLAKIIQKAHAEGVCGLRSVSRLEHQMWEAIKDELNTLLKENGVDLSVVVEKQNGMYKAAPKRLAATTEKLEMGWKAWGKSIIFAPELANNTFVIDGPETEECPTANRAWNSMEVEDFGFGLTSTRMFLRIRETNTTECDSKIIGTAVKNNMAVHSDLSYWIESGLNDTWKLERAVLGEVKSCTWPETHTLWGDGVLESDLIIPITLAGPRSNHNRRPGYKTQNQGPWDEGRVEIDFDYCPGTTVTISDSCEHRGPAARTTTESGKLITDWCCRSCTLPPLRFQTENGCWYGMEIRPTRHDEKTLVQSRVNAYNADMIDPFQLGLMVVFLATQEVLRKRWTAKISIPAIMLALLVLVFGGITYTDVLRYVILVGAAFAEANSGGDVVHLALMATFKIQPVFLVASFLKARWTNQESILLMLAAAFFQMAYYDAKNVLSWEVPDVLNSLSVAWMILRAISFTNTSNVVVPLLALLTPGLKCLNLDVYRILLLMVGVGSLIKEKRSSAAKKKGACLICLALASTGVFNPMILAAGLMACDPNRKRGWPATEVMTAVGLMFAIVGGLAELDIDSMAIPMTIAGLMFAAFVISGKSTDMWIERTADITWESDAEITGSSERVDVRLDDDGNFQLMNDPGAPWKIWMLRMACLAISAYTPWAILPSVIGFWITLQYTKRGGVLWDTPSPKEYKKGDTTTGVYRIMTRGLLGSYQAGAGVMVEGVFHTLWHTTKGAALMSGEGRLDPYWGSVKEDRLCYGGPWKLQHKWNGHDEVQMIVVEPGKNVKNVQTKPGVFKTPEGEIGAVTLDYPTGTSGSPIVDKNGDVIGLYGNGVIMPNGSYISAIVQGERMEEPAPAGFEPEMLRKKQITVLDLHPGAGKTRKILPQIIKEAINKRLRTAVLAPTRVVAAEMSEALRGLPIRYQTSAVHREHSGNEIVDVMCHATLTHRLMSPHRVPNYNLFIMDEAHFTDPASIAARGYIATKVELGEAAAIFMTATPPGTSDPFPESNAPISDMQTEIPDRAWNTGYEWITEYVGKTVWFVPSVKMGNEIALCLQRAGKKVIQLNRKSYETEYPKCKNDDWDFVITTDISEMGANFKASRVIDSRKSVKPTIIEEGDGRVILGEPSAITAASAAQRRGRIGRNPSQVGDEYCYGGHTNEDDSNFAHWTEARIMLDNINMPNGLVAQLYQPEREKVYTMDGEYRLRGEERKNFLEFLRTADLPVWLAYKVAAAGISYHDRKWCFDGPRTNTILEDNNEVEVITKLGERKILRPRWADARVYSDHQALKSFKDFASGKRSQIGLVEVLGRMPEHFMVKTWEALDTMYVVATAEKGGRAHRMALEELPDALQTIVLIALLSVMSLGVFFLLMQRKGIGKIGLGGVILGAATFFCWMAEVPGTKIAGMLLLSLLLMIVLIPEPEKQRSQTDNQLAVFLICVLTLVGAVAANEMGWLDKTKNDIGSLLGHRPEARETTLGVESFLLDLRPATAWSLYAVTTAVLTPLLKHLITSDYINTSLTSINVQASALFTLARGFPFVDVGVSALLLAVGCWGQVTLTVTVTAAALLFCHYAYMVPGWQAEAMRSAQRRTAAGIMKNVVVDGIVATDVPELERTTPVMQKKVGQIILILVSMAAVVVNPSVRTVREAGILTTAAAVTLWENGASSVWNATTAIGLCHIMRGGWLSCLSIMWTLIKNMEKPGLKRGGAKGRTLGEVWKERLNHMTKEEFTRYRKEAITEVDRSAAKHARREGNITGGHPVSRGTAKLRWLVERRFLEPVGKVVDLGCGRGGWCYYMATQKRVQEVKGYTKGGPGHEEPQLVQSYGWNIVTMKSGVDVFYRPSEASDTLLCDIGESSSSAEVEEHRTVRVLEMVEDWLHRGPKEFCIKVLCPYMPKVIEKMETLQRRYGGGLIRNPLSRNSTHEMYWVSHASGNIVHSVNMTSQVLLGRMEKKTWKGPQFEEDVNLGSGTRAVGKPLLNSDTSKIKNRIERLKKEYSSTWHQDANHPYRTWNYHGSYEVKPTGSASSLVNGVVRLLSKPWDTITNVTTMAMTDTTPFGQQRVFKEKVDTKAPEPPEGVKYVLNETTNWLWAFLARDKKPRMCSREEFIGKVNSNAALGAMFEEQNQWKNAREAVEDPKFWEMVDEEREAHLRGECNTCIYNMMGKREKKPGEFGKAKGSRAIWFMWLGARFLEFEALGFLNEDHWLGRKNSGGGVEGLGLQKLGYILKEVGTKPGGKVYADDTAGWDTRITKADLENEAKVLELLDGEHRRLARSIIELTYRHKVVKVMRPAADGKTVMDVISREDQRGSGQVVTYALNTFTNLAVQLVRMMEGEGVIGPDDVEKLGKGKGPKVRTWLFENGEERLSRMAVSGDDCVVKPLDDRFATSLHFLNAMSKVRKDIQEWKPSTGWYDWQQVPFCSNHFTELIMKDGRTLVVPCRGQDELIGRARISPGAGWNVRDTACLAKSYAQMWLLLYFHRRDLRLMANAICSAVPANWVPTGRTTWSIHAKGEWMTTEDMLAVWNRVWIEENEWMEDKTPVERWSDVPYSGKREDIWCGSLIGTRTRATWAENIHVAINQVRSVIGEEKYVDYMSSLRRYEDTIVVEDTVL
Inhibitor
Name:
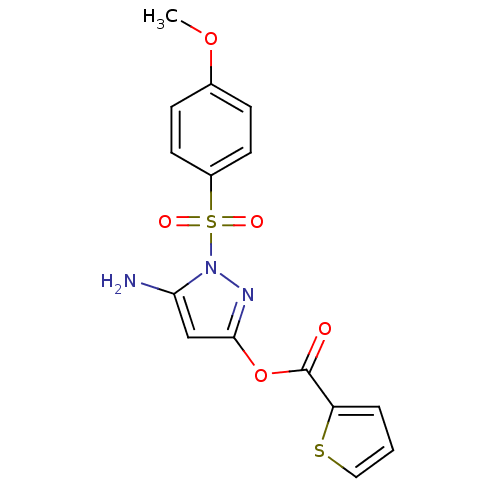
BDBM35509
Synonyms:
2-thiophenecarboxylic acid [5-amino-1-(4-methoxyphenyl)sulfonyl-3-pyrazolyl] ester | 5-amino-1-(4-methoxyphenylsulfonyl)-1H-pyrazol-3-yl thiophene-2-carboxylate | 5-amino-1-[(4-methoxyphenyl)sulfonyl]-1H-pyrazol-3-yl thiophene-2-carboxylate | CHEMBL243314 | MLS000086044 | SMR000021572 | [5-amino-1-(4-methoxyphenyl)sulfonylpyrazol-3-yl] thiophene-2-carboxylate | [5-azanyl-1-(4-methoxyphenyl)sulfonyl-pyrazol-3-yl] thiophene-2-carboxylate | cid_3240114 | thiophene-2-carboxylic acid [5-amino-1-(4-methoxyphenyl)sulfonyl-pyrazol-3-yl] ester
Type:
Small organic molecule
Emp. Form.:
C15H13N3O5S2
Mol. Mass.:
379.411
SMILES:
COc1ccc(cc1)S(=O)(=O)n1nc(OC(=O)c2cccs2)cc1N