Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Complement factor B
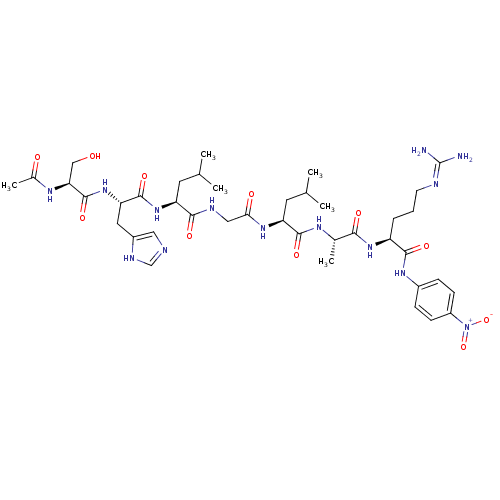
Ligand
BDBM50314035
Substrate
n/a
Meas. Tech.
ChEMBL_626636 (CHEMBL1112743)
pH
9.5±n/a
IC50
19000±n/a nM
Comments
extracted
Citation
 Ruiz-Gómez, G; Lim, J; Halili, MA; Le, GT; Madala, PK; Abbenante, G; Fairlie, DP Structure-activity relationships for substrate-based inhibitors of human complement factor B. J Med Chem 52:6042-52 (2009) [PubMed] Article
Ruiz-Gómez, G; Lim, J; Halili, MA; Le, GT; Madala, PK; Abbenante, G; Fairlie, DP Structure-activity relationships for substrate-based inhibitors of human complement factor B. J Med Chem 52:6042-52 (2009) [PubMed] Article More Info.:
Target
Name:
Complement factor B
Synonyms:
BF | BFD | CFAB_HUMAN | CFB
Type:
Enzyme
Mol. Mass.:
85537.97
Organism:
Homo sapiens (Human)
Description:
P00751
Residue:
764
Sequence:
MGSNLSPQLCLMPFILGLLSGGVTTTPWSLARPQGSCSLEGVEIKGGSFRLLQEGQALEYVCPSGFYPYPVQTRTCRSTGSWSTLKTQDQKTVRKAECRAIHCPRPHDFENGEYWPRSPYYNVSDEISFHCYDGYTLRGSANRTCQVNGRWSGQTAICDNGAGYCSNPGIPIGTRKVGSQYRLEDSVTYHCSRGLTLRGSQRRTCQEGGSWSGTEPSCQDSFMYDTPQEVAEAFLSSLTETIEGVDAEDGHGPGEQQKRKIVLDPSGSMNIYLVLDGSDSIGASNFTGAKKCLVNLIEKVASYGVKPRYGLVTYATYPKIWVKVSEADSSNADWVTKQLNEINYEDHKLKSGTNTKKALQAVYSMMSWPDDVPPEGWNRTRHVIILMTDGLHNMGGDPITVIDEIRDLLYIGKDRKNPREDYLDVYVFGVGPLVNQVNINALASKKDNEQHVFKVKDMENLEDVFYQMIDESQSLSLCGMVWEHRKGTDYHKQPWQAKISVIRPSKGHESCMGAVVSEYFVLTAAHCFTVDDKEHSIKVSVGGEKRDLEIEVVLFHPNYNINGKKEAGIPEFYDYDVALIKLKNKLKYGQTIRPICLPCTEGTTRALRLPPTTTCQQQKEELLPAQDIKALFVSEEEKKLTRKEVYIKNGDKKGSCERDAQYAPGYDKVKDISEVVTPRFLCTGGVSPYADPNTCRGDSGGPLIVHKRSRFIQVGVISWGVVDVCKNQKRQKQVPAHARDFHINLFQVLPWLKEKLQDEDLGFL
Inhibitor
Name:
BDBM50314035
Synonyms:
(S)-2-[(S)-2-((S)-2-Acetylamino-3-hydroxy-propionylamino)-3-(3H-imidazol-4-yl)-propionylamino]-4-methyl-pentanoic acid [((S)-1-{(S)-1-[(S)-4-guanidino-1-(4-nitro-phenylcarbamoyl)-butylcarbamoyl]-ethylcarbamoyl}-3-methyl-butylcarbamoyl)-methyl]-amide | Ac-SHLGLAR-pNA | CHEMBL455818
Type:
Small organic molecule
Emp. Form.:
C40H62N14O11
Mol. Mass.:
915.0075
SMILES:
CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(C)=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)Nc1ccc(cc1)[N+]([O-])=O |r,wU:4.4,32.32,45.45,18.21,wD:8.15,40.41,(2,1.83,;2,.3,;3.35,-.48,;.65,-.48,;.65,-2,;-.67,-2.76,;-2.02,-1.99,;-2.02,-.46,;-3.35,-2.74,;-3.35,-4.3,;-2.02,-5.07,;-.55,-4.67,;.29,-5.96,;-.67,-7.16,;-2.11,-6.61,;-4.68,-1.98,;-6.02,-2.74,;-6.02,-4.27,;-7.35,-1.97,;-7.35,-.41,;-6,.36,;-8.67,-2.74,;-10.01,-1.97,;-11.35,-2.74,;-10.02,-.43,;2,-2.78,;2,-4.34,;3.32,-2.02,;4.66,-2.8,;5.98,-2.04,;5.98,-.52,;7.33,-2.81,;8.65,-2.06,;8.65,-.53,;10,.25,;10,1.76,;11.36,-.53,;10,-2.83,;10,-4.4,;11.32,-2.08,;12.67,-2.86,;12.67,-4.41,;13.99,-2.1,;13.99,-.57,;15.34,-2.87,;16.65,-2.11,;16.65,-.6,;18,.19,;18,1.71,;19.35,2.48,;19.35,4.01,;18.04,4.77,;20.71,4.79,;18,-2.89,;18,-4.45,;19.32,-2.14,;20.65,-2.91,;20.64,-4.45,;21.97,-5.23,;23.31,-4.47,;23.31,-2.92,;21.98,-2.15,;24.65,-5.25,;24.64,-6.79,;25.98,-4.48,)|