Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor alpha
Ligand
BDBM50314814
Substrate
n/a
Meas. Tech.
ChEMBL_630653 (CHEMBL1111087)
EC50
8±n/a nM
Citation
 Ye, XY; Chen, S; Zhang, H; Locke, KT; O'Malley, K; Zhang, L; Srivastava, R; Miao, B; Meyers, D; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Lippy, J; Twamley, C; Muckelbauer, JK; Chang, C; An, Y; Hosagrahara, V; Zhang, L; Yang, TJ; Mukherjee, R; Cheng, PT; Tino, JA Synthesis and structure-activity relationships of 2-aryl-4-oxazolylmethoxy benzylglycines and 2-aryl-4-thiazolylmethoxy benzylglycines as novel, potent PPARalpha selective activators- PPARalpha and PPARgamma selectivity modulation. Bioorg Med Chem Lett 20:2933-7 (2010) [PubMed] Article
Ye, XY; Chen, S; Zhang, H; Locke, KT; O'Malley, K; Zhang, L; Srivastava, R; Miao, B; Meyers, D; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Lippy, J; Twamley, C; Muckelbauer, JK; Chang, C; An, Y; Hosagrahara, V; Zhang, L; Yang, TJ; Mukherjee, R; Cheng, PT; Tino, JA Synthesis and structure-activity relationships of 2-aryl-4-oxazolylmethoxy benzylglycines and 2-aryl-4-thiazolylmethoxy benzylglycines as novel, potent PPARalpha selective activators- PPARalpha and PPARgamma selectivity modulation. Bioorg Med Chem Lett 20:2933-7 (2010) [PubMed] Article More Info.:
Target
Name:
Peroxisome proliferator-activated receptor alpha
Synonyms:
NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha)
Type:
Enzyme
Mol. Mass.:
52222.08
Organism:
Homo sapiens (Human)
Description:
Q07869
Residue:
468
Sequence:
MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSCPGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACEGCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSEKAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFVIHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANLDLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFDFAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDIFLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
Inhibitor
Name:
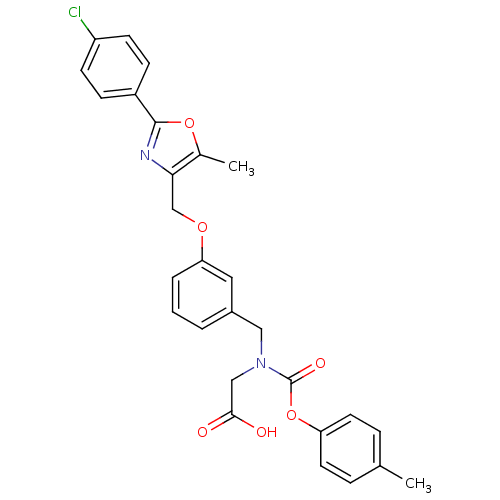
BDBM50314814
Synonyms:
2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)methoxy)benzyl)(p-tolyloxycarbonyl)amino)acetic acid | 2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)methoxy)benzyl)(ptolyloxycarbonyl)amino)acetic acid | CHEMBL1089210 | N-(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}benzyl)-N-[(4-methylphenoxy)carbonyl]glycine
Type:
Small organic molecule
Emp. Form.:
C28H25ClN2O6
Mol. Mass.:
520.961
SMILES:
Cc1oc(nc1COc1cccc(CN(CC(O)=O)C(=O)Oc2ccc(C)cc2)c1)-c1ccc(Cl)cc1