Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Aurora kinase C
Ligand
BDBM13535
Substrate
n/a
Meas. Tech.
ChEMBL_655687 (CHEMBL1244731)
Kd
1600±n/a nM
Citation
 Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 114:2984-92 (2009) [PubMed] Article
Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 114:2984-92 (2009) [PubMed] Article More Info.:
Target
Name:
Aurora kinase C
Synonyms:
AIE2 | AIK3 | AIRK3 | ARK3 | AURKC | AURKC_HUMAN | Aurora Kinase C (Aurora-C) | Aurora kinase C | Aurora kinase C (AURKC) | Aurora-C | Aurora-C/INCENP | Aurora/Ipl1-related kinase 3 | Aurora/Ipl1/Eg2 protein 2 | STK13 | Serine/threonine-protein kinase 13 | Serine/threonine-protein kinase Aurora-C
Type:
Enzyme
Mol. Mass.:
35602.43
Organism:
Homo sapiens (Human)
Description:
Amino acid residues 1-309 were expressed as His-tagged fusion protein using baculovirus expression system.
Residue:
309
Sequence:
MSSPRAVVQLGKAQPAGEELATANQTAQQPSSPAMRRLTVDDFEIGRPLGKGKFGNVYLARLKESHFIVALKVLFKSQIEKEGLEHQLRREIEIQAHLQHPNILRLYNYFHDARRVYLILEYAPRGELYKELQKSEKLDEQRTATIIEELADALTYCHDKKVIHRDIKPENLLLGFRGEVKIADFGWSVHTPSLRRKTMCGTLDYLPPEMIEGRTYDEKVDLWCIGVLCYELLVGYPPFESASHSETYRRILKVDVRFPLSMPLGARDLISRLLRYQPLERLPLAQILKHPWVQAHSRRVLPPCAQMAS
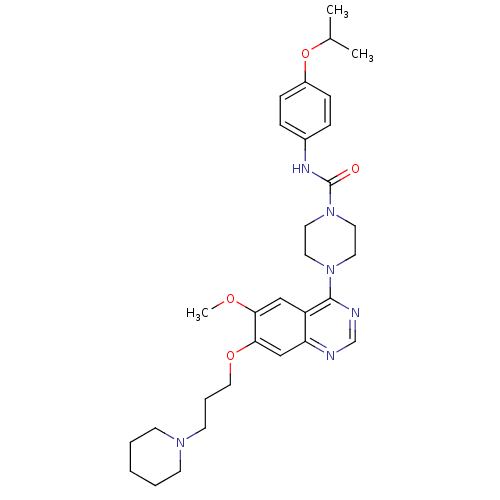
Inhibitor
Name:
BDBM13535
Synonyms:
4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-(4-propan-2-yloxyphenyl)piperazine-1-carboxamide | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-[4-(1-methylethoxy)phenyl]piperazine-1-carboxamide | 4-[6-methoxy-7-[3-(1-piperidinyl)propoxy]-4-quinazolinyl]-N-(4-propan-2-yloxyphenyl)-1-piperazinecarboxamide | 4-{6-methoxy-7-[3-(piperidin-1-yl)propoxy]quinazolin-4-yl}-N-[4-(propan-2-yloxy)phenyl]piperazine-1-carboxamide | CHEMBL124660 | MLN-518 | MLN518 | N-(4-isopropoxyphenyl)-4-[6-methoxy-7-(3-piperidinopropoxy)quinazolin-4-yl]piperazine-1-carboxamide | cid_3038522
Type:
Small organic molecule
Emp. Form.:
C31H42N6O4
Mol. Mass.:
562.703
SMILES:
COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1