Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM21398
Substrate
n/a
Meas. Tech.
ChEMBL_665580 (CHEMBL1261464)
Ki
30±n/a nM
Citation
 Skultety, M; Hübner, H; Löber, S; Gmeiner, P Bioisosteric replacement leading to biologically active [2.2]paracyclophanes with altered binding profiles for aminergic G-protein-coupled receptors. J Med Chem 53:7219-28 (2010) [PubMed] Article
Skultety, M; Hübner, H; Löber, S; Gmeiner, P Bioisosteric replacement leading to biologically active [2.2]paracyclophanes with altered binding profiles for aminergic G-protein-coupled receptors. J Med Chem 53:7219-28 (2010) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2 | 5-HT-2A | 5-HT2 | 5-HT2A | 5HT2A_PIG | HTR2A | Serotonin 2a (5-HT2a) receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
52679.13
Organism:
PIG
Description:
5-HT2 0 0::P50129
Residue:
470
Sequence:
MDVLCEENTSLSSPTNSFMQLNDDTRLYHNDFNSGEANTSDAFNWTVDSENRTNLSCEGCLSPPCFSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHRRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKSSQLQTGQKENSKQDDKATENDCTMVALGKQHSEDAPADNSNTVNEKVSCV
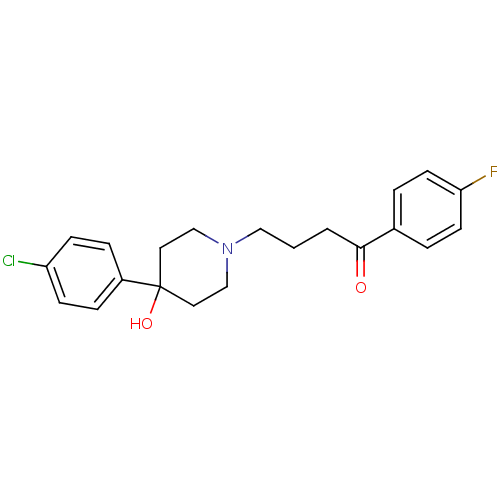
Inhibitor
Name:
BDBM21398
Synonyms:
4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-butan-1-one;propionate(HCl) | 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one | CHEMBL54 | CHEMBL545608 | Haloperidol | Haloperidol, 1
Type:
Small organic molecule
Emp. Form.:
C21H23ClFNO2
Mol. Mass.:
375.864
SMILES:
OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1