Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Renin
Ligand
BDBM50329022
Substrate
n/a
Meas. Tech.
ChEMBL_674153 (CHEMBL1274250)
IC50
175±n/a nM
Citation
 Scheiper, B; Matter, H; Steinhagen, H; Stilz, U; Böcskei, Z; Fleury, V; McCort, G Discovery and optimization of a new class of potent and non-chiral indole-3-carboxamide-based renin inhibitors. Bioorg Med Chem Lett 20:6268-72 (2010) [PubMed] Article
Scheiper, B; Matter, H; Steinhagen, H; Stilz, U; Böcskei, Z; Fleury, V; McCort, G Discovery and optimization of a new class of potent and non-chiral indole-3-carboxamide-based renin inhibitors. Bioorg Med Chem Lett 20:6268-72 (2010) [PubMed] Article More Info.:
Target
Name:
Renin
Synonyms:
Angiotensinogenase | REN | RENI_HUMAN
Type:
Enzyme
Mol. Mass.:
45058.99
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
406
Sequence:
MDGWRRMPRWGLLLLLWGSCTFGLPTDTTTFKRIFLKRMPSIRESLKERGVDMARLGPEWSQPMKRLTLGNTTSSVILTNYMDTQYYGEIGIGTPPQTFKVVFDTGSSNVWVPSSKCSRLYTACVYHKLFDASDSSSYKHNGTELTLRYSTGTVSGFLSQDIITVGGITVTQMFGEVTEMPALPFMLAEFDGVVGMGFIEQAIGRVTPIFDNIISQGVLKEDVFSFYYNRDSENSQSLGGQIVLGGSDPQHYEGNFHYINLIKTGVWQIQMKGVSVGSSTLLCEDGCLALVDTGASYISGSTSSIEKLMEALGAKKRLFDYVVKCNEGPTLPDISFHLGGKEYTLTSADYVFQESYSSKKLCTLAIHAMDIPPPTGPTWALGATFIRKFYTEFDRRNNRIGFALAR
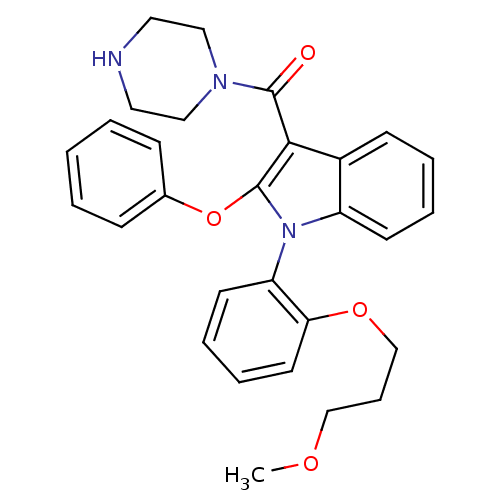
Inhibitor
Name:
BDBM50329022
Synonyms:
(1-(2-(3-methoxypropoxy)phenyl)-2-phenoxy-1H-indol-3-yl)(piperazin-1-yl)methanone | CHEMBL1271079
Type:
Small organic molecule
Emp. Form.:
C29H31N3O4
Mol. Mass.:
485.5741
SMILES:
COCCCOc1ccccc1-n1c(Oc2ccccc2)c(C(=O)N2CCNCC2)c2ccccc12 |(14.13,-12.49,;15.16,-11.34,;16.66,-11.66,;17.7,-10.52,;19.2,-10.84,;20.23,-9.7,;21.74,-10.02,;22.21,-11.48,;23.72,-11.8,;24.75,-10.65,;24.27,-9.19,;22.77,-8.87,;22.3,-7.41,;23.21,-6.16,;24.75,-6.16,;25.51,-7.49,;24.72,-8.81,;25.48,-10.15,;27.02,-10.16,;27.8,-8.82,;27.03,-7.49,;22.3,-4.9,;22.77,-3.44,;21.74,-2.29,;24.28,-3.12,;25.3,-4.26,;26.8,-3.95,;27.28,-2.48,;26.25,-1.34,;24.74,-1.65,;20.82,-5.38,;19.49,-4.62,;18.16,-5.39,;18.16,-6.93,;19.49,-7.71,;20.82,-6.93,)|