Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor gamma
Ligand
BDBM50345806
Substrate
n/a
Meas. Tech.
ChEMBL_749314 (CHEMBL1785095)
EC50
620000000±n/a nM
Citation
More Info.:
Target
Name:
Peroxisome proliferator-activated receptor gamma
Synonyms:
NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2
Type:
Nuclear Receptor
Mol. Mass.:
57613.46
Organism:
Homo sapiens (Human)
Description:
P37231
Residue:
505
Sequence:
MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSFDIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKTQLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNCRIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLRALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQEQSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLASLMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVIILSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQLLQVIKKTETDMSLHPLLQEIYKDLY
Inhibitor
Name:
BDBM50345806
Synonyms:
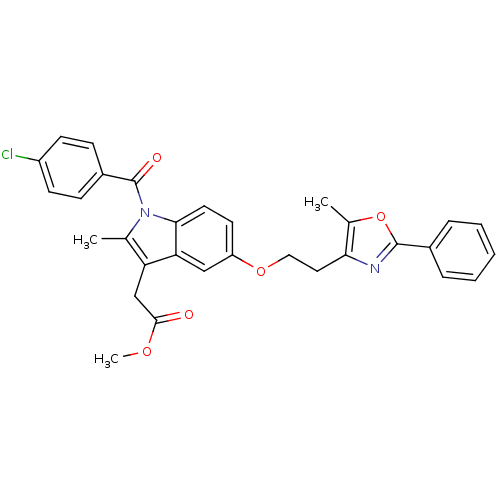
CHEMBL1784945 | methyl 2-(1-(4-chlorobenzoyl)-2-methyl-5-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-1H-indol-3-yl)acetate
Type:
Small organic molecule
Emp. Form.:
C31H27ClN2O5
Mol. Mass.:
543.009
SMILES:
COC(=O)Cc1c(C)n(C(=O)c2ccc(Cl)cc2)c2ccc(OCCc3nc(oc3C)-c3ccccc3)cc12