Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, liver form
Ligand
BDBM50136422
Substrate
n/a
Meas. Tech.
ChEMBL_762685 (CHEMBL1817503)
IC50
>20000±n/a nM
Citation
More Info.:
Target
Name:
Glycogen phosphorylase, liver form
Synonyms:
Glycogen Phosphorylase (PYGL) | Glycogen Phosphorylase, liver form | Liver glycogen phosphorylase | PYGL | PYGL_HUMAN
Type:
Homodimer
Mol. Mass.:
97153.98
Organism:
Homo sapiens (Human)
Description:
Dimers associate into a tetramer to form the enzymatically active phosphorylase A.
Residue:
847
Sequence:
MAKPLTDQEKRRQISIRGIVGVENVAELKKSFNRHLHFTLVKDRNVATTRDYYFALAHTVRDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDIEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIRDGWQVEEADDWLRYGNPWEKSRPEFMLPVHFYGKVEHTNTGTKWIDTQVVLALPYDTPVPGYMNNTVNTMRLWSARAPNDFNLRDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKASKFGSTRGAGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKLPWSKAWELTQKTFAYTNHTVLPEALERWPVDLVEKLLPRHLEIIYEINQKHLDRIVALFPKDVDRLRRMSLIEEEGSKRINMAHLCIVGSHAVNGVAKIHSDIVKTKVFKDFSELEPDKFQNKTNGITPRRWLLLCNPGLAELIAEKIGEDYVKDLSQLTKLHSFLGDDVFLRELAKVKQENKLKFSQFLETEYKVKINPSSMFDVQVKRIHEYKRQLLNCLHVITMYNRIKKDPKKLFVPRTVIIGGKAAPGYHMAKMIIKLITSVADVVNNDPMVGSKLKVIFLENYRVSLAEKVIPATDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRIDDVAALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPKQPDLFKDIINMLFYHDRFKVFADYEAYVKCQDKVSQLYMNPKAWNTMVLKNIAASGKFSSDRTIKEYAQNIWNVEPSDLKISLSNESNKVNGN
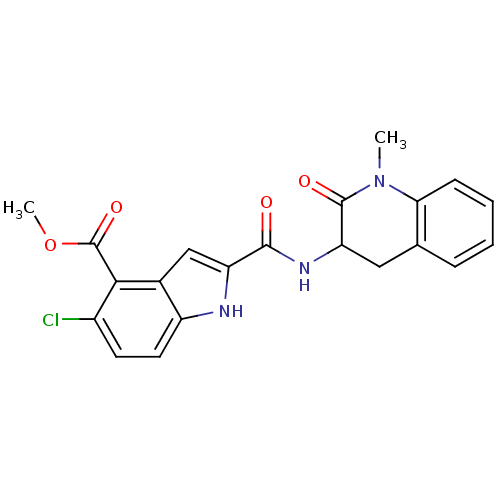
Inhibitor
Name:
BDBM50136422
Synonyms:
5-Chloro-2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-quinolin-3-ylcarbamoyl)-1H-indole-4-carboxylic acid methyl ester | CHEMBL342601
Type:
Small organic molecule
Emp. Form.:
C21H18ClN3O4
Mol. Mass.:
411.838
SMILES:
COC(=O)c1c(Cl)ccc2[nH]c(cc12)C(=O)NC1Cc2ccccc2N(C)C1=O