Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
cAMP-dependent protein kinase catalytic subunit alpha
Ligand
BDBM50161957
Substrate
n/a
Meas. Tech.
ChEMBL_774361 (CHEMBL1908578)
Kd
>10000±n/a nM
Citation
 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article More Info.:
Target
Name:
cAMP-dependent protein kinase catalytic subunit alpha
Synonyms:
KAPCA_HUMAN | PKA C-alpha | PKACA | PRKACA | cAMP-dependent protein kinase (PKA) | cAMP-dependent protein kinase catalytic (PKA) | cAMP-dependent protein kinase catalytic subunit alpha | cAMP-dependent protein kinase catalytic subunit alpha (PKA) | cAMP-dependent protein kinase catalytic subunit alpha (PKACA) | cAMP-dependent protein kinase catalytic subunit alpha (PKAc) | cAMP-dependent protein kinase catalytic subunit alpha isoform 1 | cAMP-dependent protein kinase, alpha-catalytic subunit
Type:
Enzyme Catalytic Subunit
Mol. Mass.:
40598.73
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
351
Sequence:
MGNAAAAKKGSEQESVKEFLAKAKEDFLKKWESPAQNTAHLDQFERIKTLGTGSFGRVMLVKHKETGNHYAMKILDKQKVVKLKQIEHTLNEKRILQAVNFPFLVKLEFSFKDNSNLYMVMEYVPGGEMFSHLRRIGRFSEPHARFYAAQIVLTFEYLHSLDLIYRDLKPENLLIDQQGYIQVTDFGFAKRVKGRTWTLCGTPEYLAPEIILSKGYNKAVDWWALGVLIYEMAAGYPPFFADQPIQIYEKIVSGKVRFPSHFSSDLKDLLRNLLQVDLTKRFGNLKNGVNDIKNHKWFATTDWIAIYQRKVEAPFIPKFKGPGDTSNFDDYEEEEIRVSINEKCGKEFSEF
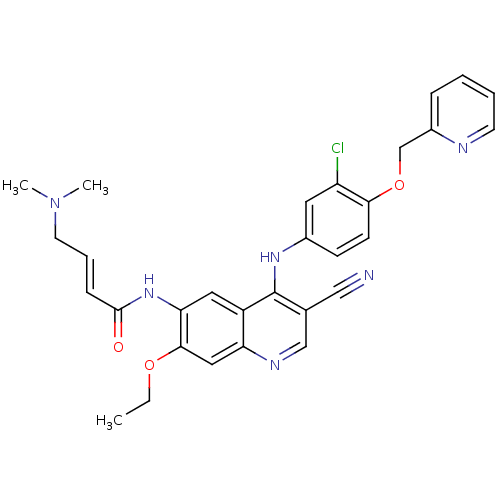
Inhibitor
Name:
BDBM50161957
Synonyms:
4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide | CHEMBL180022 | HKI-272 | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)butanamide | NERATINIB | US10822334, Compound Neratinib | US11896597, Compound Neratinib | US20230382923, Compound Neratinib
Type:
Small organic molecule
Emp. Form.:
C30H29ClN6O3
Mol. Mass.:
557.043
SMILES:
CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C