Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform
Ligand
BDBM50061067
Substrate
n/a
Meas. Tech.
ChEMBL_161791 (CHEMBL768165)
IC50
0.065000±n/a nM
Citation
More Info.:
Target
Name:
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform
Synonyms:
2ABA_HUMAN | PPP2R2A | Serine/threonine protein phosphatase 2A, 55 kDa regulatory subunit B, alpha isoform
Type:
PROTEIN
Mol. Mass.:
51688.47
Organism:
Homo sapiens (Human)
Description:
ChEMBL_161791
Residue:
447
Sequence:
MAGAGGGNDIQWCFSQVKGAVDDDVAEADIISTVEFNHSGELLATGDKGGRVVIFQQEQENKIQSHSRGEYNVYSTFQSHEPEFDYLKSLEIEEKINKIRWLPQKNAAQFLLSTNDKTIKLWKISERDKRPEGYNLKEEDGRYRDPTTVTTLRVPVFRPMDLMVEASPRRIFANAHTYHINSISINSDYETYLSADDLRINLWHLEITDRSFNIVDIKPANMEELTEVITAAEFHPNSCNTFVYSSSKGTIRLCDMRASALCDRHSKLFEEPEDPSNRSFFSEIISSISDVKFSHSGRYMMTRDYLSVKIWDLNMENRPVETYQVHEYLRSKLCSLYENDCIFDKFECCWNGSDSVVMTGSYNNFFRMFDRNTKRDITLEASRENNKPRTVLKPRKVCASGKRKKDEISVDSLDFNKKILHTAWHPKENIIAVATTNNLYIFQDKVN
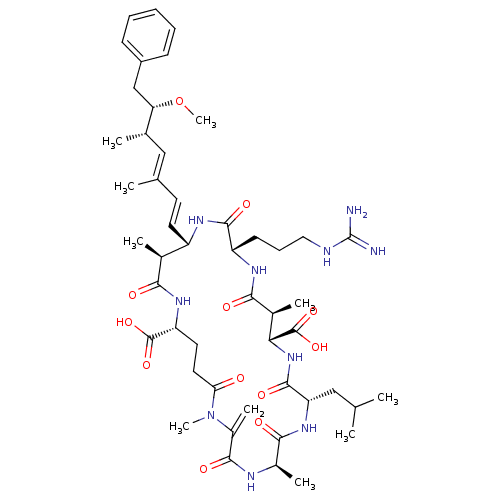
Inhibitor
Name:
BDBM50061067
Synonyms:
15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-methoxy-3,5-dimethyl-7-phenyl-hepta-1,3-dienyl)-1,5,12,19-tetramethyl-2-methylene-3,6,9,13,16,20,25-heptaoxo-1,4,7,10,14,17,21heptaaza-cyclopentacosane-11,22-dicarboxylic acid | 15-(3-Guanidino-propyl)-8-isobutyl-18-(6-methoxy-3,5-dimethyl-7-phenyl-hepta-1,3-dienyl)-1,5,12,19-tetramethyl-2-methylene-3,6,9,13,16,20,25-heptaoxo-1,4,7,10,14,17,21heptaaza-cyclopentacosane-11,22-dicarboxylic acid | 8-[3-amino(imino)methylaminopropyl]-15-isobutyl-5-[6-methoxy-3,5-dimethyl-7-phenyl-(1E,3E)-1,3-heptadienyl]-4,11,18,22-tetramethyl-21-methylene-3,7,10,14,17,20,23-heptaoxo-2,6,9,13,16,19,22-heptaazacyclopentacosane-1,12-dicarboxylic acid | CHEMBL444092 | Microcystin-LR
Type:
Small organic molecule
Emp. Form.:
C49H74N10O12
Mol. Mass.:
995.1717
SMILES:
CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O