Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosine receptor A3
Ligand
BDBM50117108
Substrate
n/a
Meas. Tech.
ChEMBL_337186 (CHEMBL862546)
Ki
0.014±n/a nM
Citation
 Pastorin, G; Da Ros, T; Bolcato, C; Montopoli, C; Moro, S; Cacciari, B; Baraldi, PG; Varani, K; Borea, PA; Spalluto, G Synthesis and biological studies of a new series of 5-heteroarylcarbamoylaminopyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidines as human A3 adenosine receptor antagonists. Influence of the heteroaryl substituent on binding affinity and molecular modeling investigations. J Med Chem 49:1720-9 (2006) [PubMed] Article
Pastorin, G; Da Ros, T; Bolcato, C; Montopoli, C; Moro, S; Cacciari, B; Baraldi, PG; Varani, K; Borea, PA; Spalluto, G Synthesis and biological studies of a new series of 5-heteroarylcarbamoylaminopyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidines as human A3 adenosine receptor antagonists. Influence of the heteroaryl substituent on binding affinity and molecular modeling investigations. J Med Chem 49:1720-9 (2006) [PubMed] Article More Info.:
Target
Name:
Adenosine receptor A3
Synonyms:
A3 adenosine receptor (hA3) | AA3R_HUMAN | ADORA3 | Adenosine A3 receptor (A3AR)
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
36197.32
Organism:
Homo sapiens (Human)
Description:
P0DMS8
Residue:
318
Sequence:
MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIAVGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKRVTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYFSFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFALSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKACVVCHPSDSLDTSIEKNSE
Inhibitor
Name:
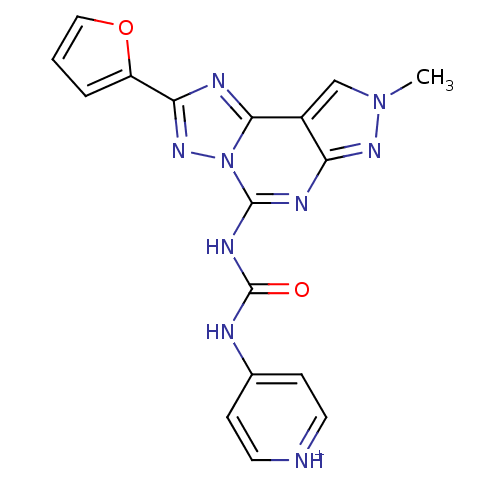
BDBM50117108
Synonyms:
4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)-ureido]-pyridinium; chloride | 5-[[(4-pyridyl)-amino]carbonyl]amino-8-methyl-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine hydrochloride | CHEMBL118923
Type:
Small organic molecule
Emp. Form.:
C17H14N9O2
Mol. Mass.:
376.3516
SMILES:
Cn1cc2c(n1)nc(NC(=O)Nc1cc[nH+]cc1)n1nc(nc21)-c1ccco1