Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenylate cyclase
Ligand
BDBM50027065
Substrate
n/a
Meas. Tech.
ChEMBL_514280 (CHEMBL979110)
IC50
70000±n/a nM
Citation
 Schlicker, C; Rauch, A; Hess, KC; Kachholz, B; Levin, LR; Buck, J; Steegborn, C Structure-based development of novel adenylyl cyclase inhibitors. J Med Chem 51:4456-64 (2008) [PubMed] Article
Schlicker, C; Rauch, A; Hess, KC; Kachholz, B; Levin, LR; Buck, J; Steegborn, C Structure-based development of novel adenylyl cyclase inhibitors. J Med Chem 51:4456-64 (2008) [PubMed] Article More Info.:
Target
Name:
Adenylate cyclase
Synonyms:
n/a
Type:
PROTEIN
Mol. Mass.:
133886.30
Organism:
Spirulina platensis
Description:
ChEMBL_514280
Residue:
1202
Sequence:
MSSPNRKLKPTILVVDDEPDNLDLLYRTFHREFKVLKAESGPAALKILEEVGEVAVIISDQRMPYMSGTEFLSLTATQYPDSIRIILTGYTDVEDLVEAINSGKVFKYVTKPWKSDELKAIVQQGLETHNVLKSRTEELRLAQKQESLLYEVTSTIRACPNSQEMLQRIVETVGKMFEVSYCLLRSFGVGSDLIGLGAGVSPTKQDITATQGKEWFAYLAEGQNHQNSTTDNISVINNNDLELRSLVWETTEVMILSEGLGNDISDHDGPEWQQRRDVYQRADIRSSLIVPLYYRQELLAVLALHHTGSPRNWHEHEVQLAAGVADQAALALSQVRAYEQVRELARREALVNTITNAIRSSLDPQKIFAAITEQLGEALEVDGCALSLWSPGDEYMQCVGLYNAAIKETVVETRPAALSEPDTSTTTNLPLLGVETNQSIESDQSDDLPQSAAPISGNPVLQELIRTRAPVAIADIEQRPDSMVMLPLRSPSKALLVVPLLLDGDIIGSISLRQNHQVRHWQPSEIDMVLLVAAQAALAVQQARLYQKTRQQAERLLEADRLKTEFFQNVSHEFRTPLTLMIGPLETVVNQQQDLSLDQAKIALRNSRRLLRLVNQLLDLQRFDAGRMQPSFRPCDLVEFCQQTVESFKSYCDRKQINLVTNLQSCPQLYLDLERFDKVLYNLLSNAMKFTPTDGTITVSLQPEGNYCRLMVKDTGIGIKQEQLPHLFERFRQAEGSANRSFEGSGLGLALVKELVELHHGRITVESEYGQGTTFTVWLQMGNLHLPPSPLLDVPAEFDARRAAVELADVEVDLPDVQIDDINLPEVLVADGSASLTDHGQLGSNTVLVVDDNPDLRRYVSMMLQNAGFNAVLAKNGADGFNKAQTYHPDVIVTDLMMPQVSGLELIRMIRSSPELRGTPIILLTAKADEDTRIEGVERGADAYVSKPFNDRELIAEVRNLQALKAEERRVAHLNKYLTESVLRRFLPESMVKKAAAGDLTLDLRPEPRLITILFSDIVGFTRMSNALQSQGVAELLNEYLGEMTRAVFENQGTVDKFVGDAIMALYGAPEEMSPSEQVRRAIATARQMLVALEKLNQGWQERGLVGRNEVPPVRFRCGIHQGMAVVGLFGSQERSDFTAIGPSVNIAARLQEATAPNSIMVSAMVAQYVPDEEIIKREFLELKGIDEPVMTCVINPNMLNQ
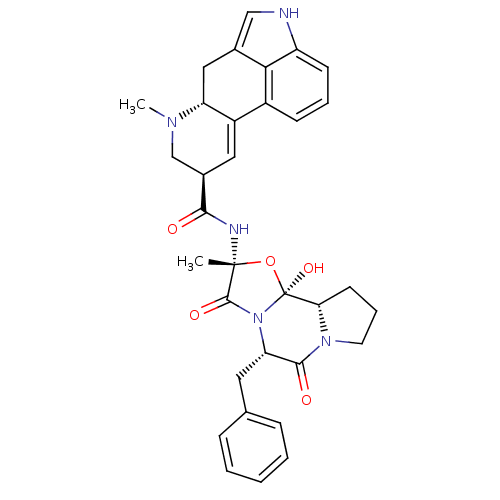
Inhibitor
Name:
BDBM50027065
Synonyms:
(5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)ergotoman-3',6',18-trione | (5'alpha)-5'-benzyl-12'-hydroxy-2'-methyl-3',6',18-trioxoergotaman | 12'-hydroxy-2'-methyl-5'alpha-(phenylmethyl)ergotaman-3',6',18-trione | ERGOTAMINE | Ergotamin
Type:
Small organic molecule
Emp. Form.:
C33H35N5O5
Mol. Mass.:
581.6615
SMILES:
CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4|