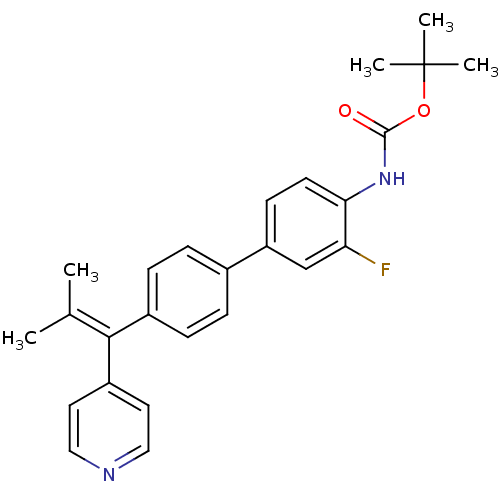
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Steroid 17-alpha-hydroxylase/17,20 lyase
Ligand
BDBM50322800
Substrate
n/a
Meas. Tech.
ChEMBL_642391 (CHEMBL1177584)
IC50
852±n/a nM
Citation
 Hu, Q; Yin, L; Jagusch, C; Hille, UE; Hartmann, RW Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J Med Chem 53:5049-53 (2010) [PubMed] Article
Hu, Q; Yin, L; Jagusch, C; Hille, UE; Hartmann, RW Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J Med Chem 53:5049-53 (2010) [PubMed] Article More Info.:
Target
Name:
Steroid 17-alpha-hydroxylase/17,20 lyase
Synonyms:
CP17A_HUMAN | CYP17 | CYP17A1 | CYPXVII | Cytochrome P450 17A1 | Cytochrome P450 C17 (CYP17 ) | Cytochrome P450 C17 (CYP17) | Cytochrome P450 CYP17 | Cytochrome P450-C17 | Cytochrome P450-C17 (CYP17) | P450-C17 | S17AH | Steroid 17-alpha-Monooxygenase (CYP17) | Steroid 17-alpha-hydroxylase/17,20 lyase | Steroid 17-alpha-monooxygenase | cytochrome P450 monooxygenase 17 alpha hydroxylase/17,20-lyase (CYP17)
Type:
Enzyme
Mol. Mass.:
57382.42
Organism:
Homo sapiens (Human)
Description:
E.coli expressing human CYP17
Residue:
508
Sequence:
MWELVALLLLTLAYLFWPKRRCPGAKYPKSLLSLPLVGSLPFLPRHGHMHNNFFKLQKKYGPIYSVRMGTKTTVIVGHHQLAKEVLIKKGKDFSGRPQMATLDIASNNRKGIAFADSGAHWQLHRRLAMATFALFKDGDQKLEKIICQEISTLCDMLATHNGQSIDISFPVFVAVTNVISLICFNTSYKNGDPELNVIQNYNEGIIDNLSKDSLVDLVPWLKIFPNKTLEKLKSHVKIRNDLLNKILENYKEKFRSDSITNMLDTLMQAKMNSDNGNAGPDQDSELLSDNHILTTIGDIFGAGVETTTSVVKWTLAFLLHNPQVKKKLYEEIDQNVGFSRTPTISDRNRLLLLEATIREVLRLRPVAPMLIPHKANVDSSIGEFAVDKGTEVIINLWALHHNEKEWHQPDQFMPERFLNPAGTQLISPSVSYLPFGAGPRSCIGEILARQELFLIMAWLLQRFDLEVPDDGQLPSLEGIPKVVFLIDSFKVKIKVRQAWREAQAEGST
Inhibitor
Name:
BDBM50322800
Synonyms:
CHEMBL1173795 | tert-butyl 3-fluoro-4'-(2-methyl-1-(pyridin-4-yl)prop-1-enyl)biphenyl-4-ylcarbamate
Type:
Small organic molecule
Emp. Form.:
C26H27FN2O2
Mol. Mass.:
418.5032
SMILES:
[#6]\[#6](-[#6])=[#6](/c1ccncc1)-c1ccc(cc1)-c1ccc(-[#7]-[#6](=O)-[#8]C([#6])([#6])[#6])c(F)c1