Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Maltase-glucoamylase
Ligand
BDBM50330955
Substrate
n/a
Meas. Tech.
ChEMBL_684093 (CHEMBL1286470)
Ki
1000±n/a nM
Citation
 Mohan, S; Sim, L; Rose, DR; Pinto, BM Probing the active-site requirements of human intestinal N-terminal maltase-glucoamylase: Synthesis and enzyme inhibitory activities of a six-membered ring nitrogen analogue of kotalanol and its de-O-sulfonated derivative. Bioorg Med Chem 18:7794-8 (2010) [PubMed] Article
Mohan, S; Sim, L; Rose, DR; Pinto, BM Probing the active-site requirements of human intestinal N-terminal maltase-glucoamylase: Synthesis and enzyme inhibitory activities of a six-membered ring nitrogen analogue of kotalanol and its de-O-sulfonated derivative. Bioorg Med Chem 18:7794-8 (2010) [PubMed] Article More Info.:
Target
Name:
Maltase-glucoamylase
Synonyms:
Alpha glucosidase | Alpha-1,4-glucosidase | Glucan 1,4-alpha-glucosidase | MGA | MGAM | MGAML | MGA_HUMAN | Maltase | Maltase-glucoamylase, intestinal | Synonyms=MGA
Type:
Enzyme
Mol. Mass.:
209817.06
Organism:
Homo sapiens (Human)
Description:
O43451
Residue:
2753
Sequence:
MARKKLKKFTTLEIVLSVLLLVLFIISIVLIVLLAKESLKSTAPDPGTTGTPDPGTTGTPDPGTTGTTHARTTGPPDPGTTGTTPVSAECPVVNELERINCIPDQPPTKATCDQRGCCWNPQGAVSVPWCYYSKNHSYHVEGNLVNTNAGFTARLKNLPSSPVFGSNVDNVLLTAEYQTSNRFHFKLTDQTNNRFEVPHEHVQSFSGNAAASLTYQVEISRQPFSIKVTRRSNNRVLFDSSIGPLLFADQFLQLSTRLPSTNVYGLGEHVHQQYRHDMNWKTWPIFNRDTTPNGNGTNLYGAQTFFLCLEDASGLSFGVFLMNSNAMEVVLQPAPAITYRTIGGILDFYVFLGNTPEQVVQEYLELIGRPALPSYWALGFHLSRYEYGTLDNMREVVERNRAAQLPYDVQHADIDYMDERRDFTYDSVDFKGFPEFVNELHNNGQKLVIIVDPAISNNSSSSKPYGPYDRGSDMKIWVNSSDGVTPLIGEVWPGQTVFPDYTNPNCAVWWTKEFELFHNQVEFDGIWIDMNEVSNFVDGSVSGCSTNNLNNPPFTPRILDGYLFCKTLCMDAVQHWGKQYDIHNLYGYSMAVATAEAAKTVFPNKRSFILTRSTFAGSGKFAAHWLGDNTATWDDLRWSIPGVLEFNLFGIPMVGPDICGFALDTPEELCRRWMQLGAFYPFSRNHNGQGYKDQDPASFGADSLLLNSSRHYLNIRYTLLPYLYTLFFRAHSRGDTVARPLLHEFYEDNSTWDVHQQFLWGPGLLITPVLDEGAEKVMAYVPDAVWYDYETGSQVRWRKQKVEMELPGDKIGLHLRGGYIFPTQQPNTTTLASRKNPLGLIIALDENKEAKGELFWDNGETKDTVANKVYLLCEFSVTQNRLEVNISQSTYKDPNNLAFNEIKILGTEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDIDLLLGEAYTVEWSIKIRDEEKIDCYPDENGASAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVYANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNKNRYEVPVPLNIPSMPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFSDMFIRISTRLPSKYLYGFGETEHRSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEIASLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPHLESRDRGLSSKTLCMESQQILPDGSLVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDAAFVNISRNVLQTRYTLLPYLYTLMQKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRKNPLGLIIALDENKEAKGELFWDDGQTKDTVAKKVYLLCEFSVTQNHLEVTISQSTYKDPNNLAFNEIKILGMEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDINLFLGEAYTVEWSIKIRDEEKIDCYPDENGDSAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVHANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNNNRYEVPVPLNIPSVPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFNDMFIRISTRLPSKYLYGFGETEHTSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEISSLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPYLESRDRGLSSKTLCMESQQILPDGSPVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDVAFVNISRTVLQTRYTLLPYLYTLMHKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRQKFMGFKIALDDEGTAGGWLFWDDGQSIDTYGKGLYYLASFSASQNTMQSHIIFNNYITGTNPLKLGYIEIWGVGSVPVTSVSISVSGMVITPSFNNDPTTQVLSIDVTDRNISLHNFTSLTWISTL
Inhibitor
Name:
BDBM50330955
Synonyms:
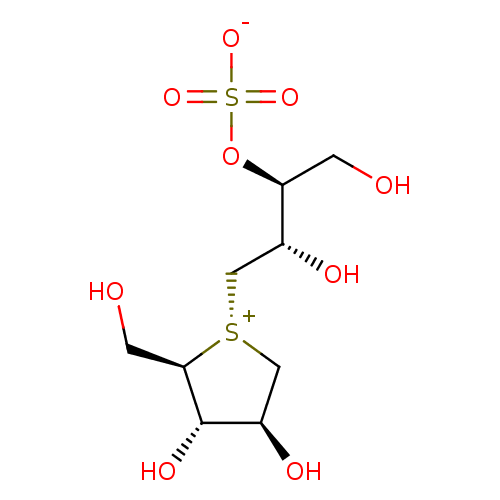
(1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)tetrahydrothiophenium-1-yl]-2-hydroxy-1-(hydroxymethyl)propyl sulfate | 1,4-dideoxy-1,4-{(S)-[(2S,3S)-2,4-dihydroxy-3-(sulfooxy)butyl]episulfoniumylidene}-D-arabinitol | CHEMBL1208974 | salacinol
Type:
Small organic molecule
Emp. Form.:
C9H18O9S2
Mol. Mass.:
334.364
SMILES:
OC[C@H](OS([O-])(=O)=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r|