Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50379595
Substrate
n/a
Meas. Tech.
ChEMBL_811669 (CHEMBL2014182)
IC50
700±n/a nM
Citation
 Wu, L; Lu, K; Packiarajan, M; Jubian, V; Chandrasena, G; Wolinsky, TC; Walker, MW Indolyl and dihydroindolyl N-glycinamides as potent and in vivo active NPY5 antagonists. Bioorg Med Chem Lett 22:2167-71 (2012) [PubMed] Article
Wu, L; Lu, K; Packiarajan, M; Jubian, V; Chandrasena, G; Wolinsky, TC; Walker, MW Indolyl and dihydroindolyl N-glycinamides as potent and in vivo active NPY5 antagonists. Bioorg Med Chem Lett 22:2167-71 (2012) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
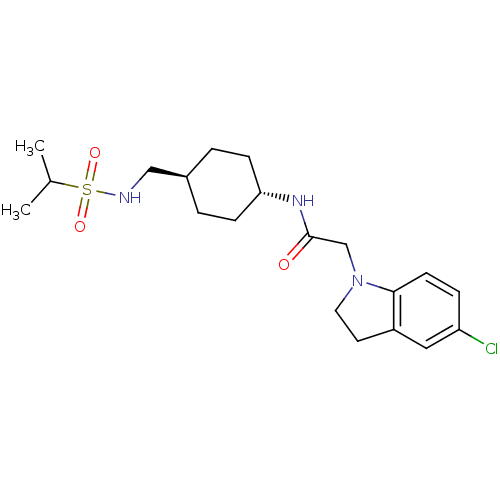
Inhibitor
Name:
BDBM50379595
Synonyms:
CHEMBL2013012
Type:
Small organic molecule
Emp. Form.:
C20H30ClN3O3S
Mol. Mass.:
427.989
SMILES:
CC(C)S(=O)(=O)NC[C@H]1CC[C@@H](CC1)NC(=O)CN1CCc2cc(Cl)ccc12 |r,wU:8.7,wD:11.14,(11.13,-18.2,;9.8,-17.43,;9.8,-15.89,;8.46,-18.19,;9.22,-19.52,;7.69,-19.52,;7.13,-17.43,;5.8,-18.19,;4.46,-17.42,;3.13,-18.19,;1.8,-17.42,;1.8,-15.88,;3.13,-15.1,;4.46,-15.88,;.47,-15.12,;-.86,-15.89,;-.86,-17.43,;-2.2,-15.12,;-3.53,-15.9,;-3.69,-17.43,;-5.2,-17.75,;-5.97,-16.42,;-7.47,-16.11,;-7.95,-14.65,;-9.46,-14.34,;-6.92,-13.5,;-5.42,-13.82,;-4.94,-15.28,)|