Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Telomerase reverse transcriptase
Ligand
BDBM50105463
Substrate
n/a
Meas. Tech.
ChEMBL_1290503 (CHEMBL3119794)
IC50
2100±n/a nM
Citation
More Info.:
Target
Name:
Telomerase reverse transcriptase
Synonyms:
EST2 | TCS1 | TERT | TERT_HUMAN | TRT
Type:
PROTEIN
Mol. Mass.:
127099.03
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1447029
Residue:
1132
Sequence:
MPRAPRCRAVRSLLRSHYREVLPLATFVRRLGPQGWRLVQRGDPAAFRALVAQCLVCVPWDARPPPAAPSFRQVSCLKELVARVLQRLCERGAKNVLAFGFALLDGARGGPPEAFTTSVRSYLPNTVTDALRGSGAWGLLLRRVGDDVLVHLLARCALFVLVAPSCAYQVCGPPLYQLGAATQARPPPHASGPRRRLGCERAWNHSVREAGVPLGLPAPGARRRGGSASRSLPLPKRPRRGAAPEPERTPVGQGSWAHPGRTRGPSDRGFCVVSPARPAEEATSLEGALSGTRHSHPSVGRQHHAGPPSTSRPPRPWDTPCPPVYAETKHFLYSSGDKEQLRPSFLLSSLRPSLTGARRLVETIFLGSRPWMPGTPRRLPRLPQRYWQMRPLFLELLGNHAQCPYGVLLKTHCPLRAAVTPAAGVCAREKPQGSVAAPEEEDTDPRRLVQLLRQHSSPWQVYGFVRACLRRLVPPGLWGSRHNERRFLRNTKKFISLGKHAKLSLQELTWKMSVRDCAWLRRSPGVGCVPAAEHRLREEILAKFLHWLMSVYVVELLRSFFYVTETTFQKNRLFFYRKSVWSKLQSIGIRQHLKRVQLRELSEAEVRQHREARPALLTSRLRFIPKPDGLRPIVNMDYVVGARTFRREKRAERLTSRVKALFSVLNYERARRPGLLGASVLGLDDIHRAWRTFVLRVRAQDPPPELYFVKVDVTGAYDTIPQDRLTEVIASIIKPQNTYCVRRYAVVQKAAHGHVRKAFKSHVSTLTDLQPYMRQFVAHLQETSPLRDAVVIEQSSSLNEASSGLFDVFLRFMCHHAVRIRGKSYVQCQGIPQGSILSTLLCSLCYGDMENKLFAGIRRDGLLLRLVDDFLLVTPHLTHAKTFLRTLVRGVPEYGCVVNLRKTVVNFPVEDEALGGTAFVQMPAHGLFPWCGLLLDTRTLEVQSDYSSYARTSIRASLTFNRGFKAGRNMRRKLFGVLRLKCHSLFLDLQVNSLQTVCTNIYKILLLQAYRFHACVLQLPFHQQVWKNPTFFLRVISDTASLCYSILKAKNAGMSLGAKGAAGPLPSEAVQWLCHQAFLLKLTRHRVTYVPLLGSLRTAQTQLSRKLPGTTLTALEAAANPALPSDFKTILD
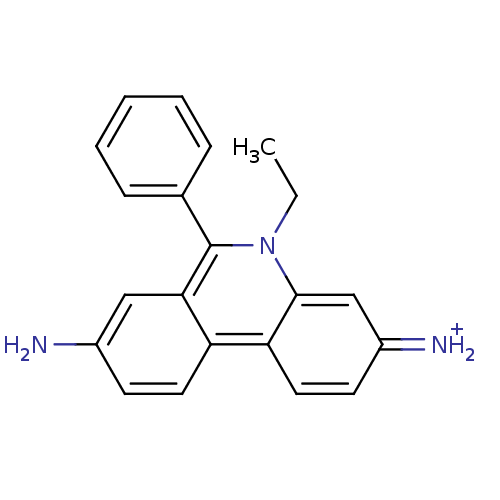
Inhibitor
Name:
BDBM50105463
Synonyms:
2,7-diamino-10-ethyl-9-phenylphenanthridinium bromide | 2,7-diamino-9-phenyl-10-ethylphenanthridinium bromide | 3,8-diamino-5-ethyl-6-phenylphenanthridinium bromide | CHEMBL284328 | Dromilac | EtBr | Ethidium bromide | Homidium bromide
Type:
Small organic molecule
Emp. Form.:
C21H20N3
Mol. Mass.:
314.4031
SMILES:
CCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12