Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
P2Y purinoceptor 12
Ligand
BDBM50100244
Substrate
n/a
Meas. Tech.
ChEMBL_1454020 (CHEMBL3367079)
IC50
150±n/a nM
Citation
 Boldron, C; Besse, A; Bordes, MF; Tissandié, S; Yvon, X; Gau, B; Badorc, A; Rousseaux, T; Barré, G; Meneyrol, J; Zech, G; Nazare, M; Fossey, V; Pflieger, AM; Bonnet-Lignon, S; Millet, L; Briot, C; Dol, F; Hérault, JP; Savi, P; Lassalle, G; Delesque, N; Herbert, JM; Bono, F N-[6-(4-butanoyl-5-methyl-1H-pyrazol-1-yl)pyridazin-3-yl]-5-chloro-1-[2-(4-methylpiperazin-1-yl)-2-oxoethyl]-1H-indole-3-carboxamide (SAR216471), a novel intravenous and oral, reversible, and directly acting P2Y12 antagonist. J Med Chem 57:7293-316 (2014) [PubMed] Article
Boldron, C; Besse, A; Bordes, MF; Tissandié, S; Yvon, X; Gau, B; Badorc, A; Rousseaux, T; Barré, G; Meneyrol, J; Zech, G; Nazare, M; Fossey, V; Pflieger, AM; Bonnet-Lignon, S; Millet, L; Briot, C; Dol, F; Hérault, JP; Savi, P; Lassalle, G; Delesque, N; Herbert, JM; Bono, F N-[6-(4-butanoyl-5-methyl-1H-pyrazol-1-yl)pyridazin-3-yl]-5-chloro-1-[2-(4-methylpiperazin-1-yl)-2-oxoethyl]-1H-indole-3-carboxamide (SAR216471), a novel intravenous and oral, reversible, and directly acting P2Y12 antagonist. J Med Chem 57:7293-316 (2014) [PubMed] Article More Info.:
Target
Name:
P2Y purinoceptor 12
Synonyms:
P2Y purinoceptor 12 | P2Y12_RAT | P2ry12 | P2y12 | Purinergic receptor P2Y12
Type:
PROTEIN
Mol. Mass.:
39068.50
Organism:
Rattus norvegicus
Description:
ChEMBL_1454020
Residue:
343
Sequence:
MEVPGANATSANTTSIPGTSTLCSRDYKITQVLFPLLYTVLFFAGLITNSLAMRIFFQIRSKSNFIIFLKNTVISDLLMILTFPFKILSDAKLGAGHLRTLVCQVTSVTFYFTMYISISFLGLITIDRYLKTTRPFKTSSPSNLLGAKILSVAIWAFMFLLSLPNMILTNRRPKDKDITKCSFLKSEFGLVWHEIVNYICQVIFWINFLIVIVCYSLITKELYRSYVRTRGSAKAPKKRVNIKVFIIIAVFFICFVPFHFARIPYTLSQTRAVFDCNAENTLFYVKESTLWLTSLNACLDPFIYFFLCKSFRNSLMSMLRCSTSGANKKKGQEGGDPSEETPM
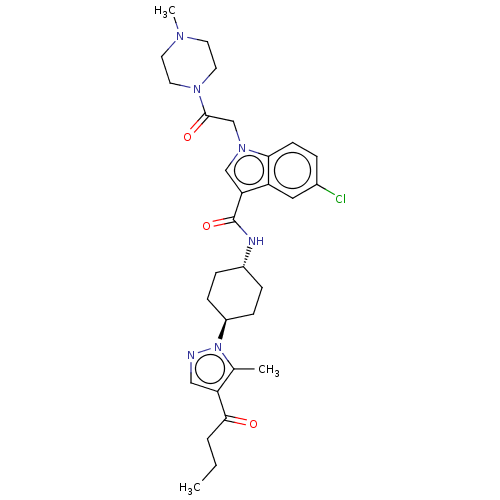
Inhibitor
Name:
BDBM50100244
Synonyms:
CHEMBL3325893
Type:
Small organic molecule
Emp. Form.:
C30H39ClN6O3
Mol. Mass.:
567.122
SMILES:
CCCC(=O)c1cnn([C@H]2CC[C@@H](CC2)NC(=O)c2cn(CC(=O)N3CCN(C)CC3)c3ccc(Cl)cc23)c1C |r,wU:12.15,wD:9.8,(-7.06,-5.13,;-5.65,-4.5,;-4.4,-5.4,;-3,-4.78,;-2.85,-3.25,;-1.75,-5.68,;-1.75,-7.22,;-.29,-7.7,;.62,-6.45,;2.16,-6.45,;2.93,-7.79,;4.47,-7.79,;5.24,-6.45,;4.47,-5.12,;2.93,-5.12,;6.78,-6.45,;7.48,-5.08,;6.64,-3.79,;9.02,-5,;9.99,-6.2,;11.43,-5.65,;12.72,-6.48,;12.72,-8.02,;11.38,-8.79,;14.05,-8.79,;15.38,-8.02,;16.72,-8.79,;16.72,-10.33,;18.05,-11.1,;15.38,-11.1,;14.05,-10.33,;11.35,-4.11,;12.43,-3.02,;12.04,-1.53,;10.55,-1.13,;10.15,.36,;9.46,-2.22,;9.86,-3.71,;-.29,-5.21,;.19,-3.74,)|