Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bile salt export pump
Ligand
BDBM31688
Substrate
n/a
Meas. Tech.
ChEMBL_1481883 (CHEMBL3540574)
IC50
11400±n/a nM
Citation
More Info.:
Target
Name:
Bile salt export pump
Synonyms:
ABCBB_RAT | ATP-binding cassette sub-family B member 11 | Abcb11 | Bile Salt Export Pump, BSEP | Bile salt export pump | Bsep | Sister of P-glycoprotein | Spgp
Type:
PROTEIN
Mol. Mass.:
146267.86
Organism:
Rattus norvegicus
Description:
ChEMBL_1481883
Residue:
1321
Sequence:
MSDSVILRSVKKFGEENHAFESDGSHNNDKKSRLQDKMKEGDIRVGFFELFRFSSSKDIWLMLMGGVCALLHGMAQPGILIIFGIMTDIFIKYDIERQELEIPGKACVNNTIVWINSSFHQNMTNGTVCGLVDIESEMIKFSGIYAGVGMTVLILGYFQIRLWVITGARQIRRMRKIYFRRIMRMEIGWFDCTSVGELNSRFADDIEKINDAIADQLAHFLQRMSTAMCGLLLGFYRGWKLTLVILAVSPLIGIGAAVIGLSIAKFTELELKAYAKAGSIADEVLSSIRTVAAFGGENKEVERYEKNLVFAQRWGIWKGMVMGFFTGYMWCLIFFCYALAFWYGSTLVLDEEEYTPGTLVQIFLCVILAAMNIGHASSCLEIFSTGCSAATNIFQTIDRQPVIDCMSGDGYKLDRIKGEIEFHNVTFHYPSRPDVKILDNLSMVIKPGETTALVGSSGAGKSTALQLIQRFYDPCEGMVTLDGHDIRSLNIRWLRDQIGIVEQEPVLFSTTIAENIRFGREDATMEDIVQAAKDANAYNFIMALPQQFDTLVGEGGGQMSGGQKQRVAIARALIRNPKILLLDMATSALDNESEARVQEALNKIQHGHTIISVAHRLSTVRAADVIIGFEHGVAVERGTHEELLERKGVYFMLVTLQSQGDNAHKETSIMGKDATEGGTLERTFSRGSYRDSLRASIRQRSKSQLSLLTHDPPLAVADHKSSYKDSKDNDVLVEEVEPAPVRRILKYNIPEWHYILVGSLSAAINGAVTPIYSLLFSQLLGTFSLLDKEQQRSEIHSMCLFFVILGCVSIFTQFLQGYTFAKSGELLTKRLRKFGFKAMLGQDIGWFDDLRNNPGVLTTRLATDASQVQGATGSQVGMMVNSFTNIIAALLIAFFFSWKLSLIITIFFPFLALSGAVQTKMLTGFASQDKQALEKAGQITSEALSNIRTVAGIGVEGRFIKAFEVELQTSYKTAVRKANIYGLCFAFSQGIAFLANSAAYRYGGYLIAYEGLGFSHVFRVVSSVALSATAVGRTFSYTPSYAKAKISAARFFQLLDRKPPINVYSEAGEKWDNFQGKIDFIDCKFTYPSRPDIQVLNGLSVSVNPGQTLAFVGSSGCGKSTSIQLLERFYDPDQGTVMIDGHDSKKVNIQFLRSNIGIVSQEPVLFDCSIMDNIKYGDNTKEISVERAIAAAKQAQLHDFVMSLPEKYETNVGIQGSQLSRGEKQRIAIARAIVRDPKILLLDEATSALDTESEKTVQTALDKAREGRTCIVIAHRLSTIQNSDIIAVVSQGVVIEKGTHEKLMAQKGAYYKLVITGAPIS
Inhibitor
Name:
BDBM31688
Synonyms:
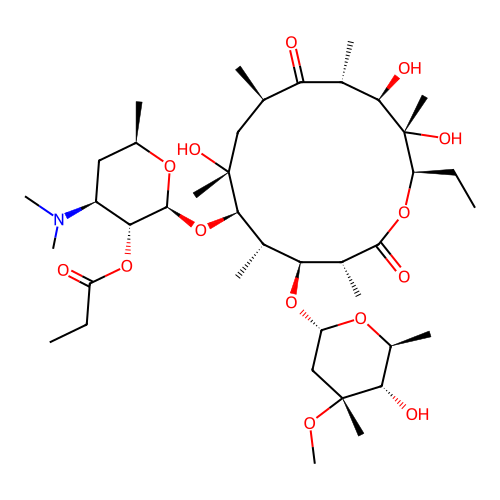
Erythromycin Estolate
Type:
macrolide antibiotic
Emp. Form.:
C52H97NO18S
Mol. Mass.:
1056.387
SMILES:
CCCCCCCCCCCCOS(O)(=O)=O.CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2OC(=O)CC)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O