Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Voltage-dependent L-type calcium channel subunit alpha-1C
Ligand
BDBM50092645
Substrate
n/a
Meas. Tech.
ChEMBL_1554471 (CHEMBL3767768)
IC50
1700±n/a nM
Citation
 Edmondson, SD; Zhu, C; Kar, NF; Di Salvo, J; Nagabukuro, H; Sacre-Salem, B; Dingley, K; Berger, R; Goble, SD; Morriello, G; Harper, B; Moyes, CR; Shen, DM; Wang, L; Ball, R; Fitzmaurice, A; Frenkl, T; Gichuru, LN; Ha, S; Hurley, AL; Jochnowitz, N; Levorse, D; Mistry, S; Miller, RR; Ormes, J; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Green, S; Struthers, M; Weber, AE Discovery of Vibegron: A Potent and Selective▀3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. J Med Chem 59:609-23 (2016) [PubMed] Article
Edmondson, SD; Zhu, C; Kar, NF; Di Salvo, J; Nagabukuro, H; Sacre-Salem, B; Dingley, K; Berger, R; Goble, SD; Morriello, G; Harper, B; Moyes, CR; Shen, DM; Wang, L; Ball, R; Fitzmaurice, A; Frenkl, T; Gichuru, LN; Ha, S; Hurley, AL; Jochnowitz, N; Levorse, D; Mistry, S; Miller, RR; Ormes, J; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Green, S; Struthers, M; Weber, AE Discovery of Vibegron: A Potent and Selective▀3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. J Med Chem 59:609-23 (2016) [PubMed] Article More Info.:
Target
Name:
Voltage-dependent L-type calcium channel subunit alpha-1C
Synonyms:
CAC1C_HUMAN | CACH2 | CACN2 | CACNA1C | CACNL1A1 | CCHL1A1 | Calcium channel (Type L) | Calcium channel, L type, alpha-1 polypeptide, isoform 1, cardiac muscle | L-type calcium channel alpha-1c/beta-2/alpha2delta-1 | Voltage-dependent L-type calcium channel subunit alpha-1C | Voltage-gated L-type calcium channel | Voltage-gated L-type calcium channel alpha-1C subunit | Voltage-gated calcium channel | Voltage-gated calcium channel subunit alpha Cav1.2
Type:
Enzyme Catalytic Domain
Mol. Mass.:
248979.79
Organism:
Homo sapiens (Human)
Description:
Calcium channel (Type L) 0 HUMAN::Q13936
Residue:
2221
Sequence:
MVNENTRMYIPEENHQGSNYGSPRPAHANMNANAAAGLAPEHIPTPGAALSWQAAIDAARQAKLMGSAGNATISTVSSTQRKRQQYGKPKKQGSTTATRPPRALLCLTLKNPIRRACISIVEWKPFEIIILLTIFANCVALAIYIPFPEDDSNATNSNLERVEYLFLIIFTVEAFLKVIAYGLLFHPNAYLRNGWNLLDFIIVVVGLFSAILEQATKADGANALGGKGAGFDVKALRAFRVLRPLRLVSGVPSLQVVLNSIIKAMVPLLHIALLVLFVIIIYAIIGLELFMGKMHKTCYNQEGIADVPAEDDPSPCALETGHGRQCQNGTVCKPGWDGPKHGITNFDNFAFAMLTVFQCITMEGWTDVLYWVNDAVGRDWPWIYFVTLIIIGSFFVLNLVLGVLSGEFSKEREKAKARGDFQKLREKQQLEEDLKGYLDWITQAEDIDPENEDEGMDEEKPRNMSMPTSETESVNTENVAGGDIEGENCGARLAHRISKSKFSRYWRRWNRFCRRKCRAAVKSNVFYWLVIFLVFLNTLTIASEHYNQPNWLTEVQDTANKALLALFTAEMLLKMYSLGLQAYFVSLFNRFDCFVVCGGILETILVETKIMSPLGISVLRCVRLLRIFKITRYWNSLSNLVASLLNSVRSIASLLLLLFLFIIIFSLLGMQLFGGKFNFDEMQTRRSTFDNFPQSLLTVFQILTGEDWNSVMYDGIMAYGGPSFPGMLVCIYFIILFICGNYILLNVFLAIAVDNLADAESLTSAQKEEEEEKERKKLARTASPEKKQELVEKPAVGESKEEKIELKSITADGESPPATKINMDDLQPNENEDKSPYPNPETTGEEDEEEPEMPVGPRPRPLSELHLKEKAVPMPEASAFFIFSSNNRFRLQCHRIVNDTIFTNLILFFILLSSISLAAEDPVQHTSFRNHILFYFDIVFTTIFTIEIALKILGNADYVFTSIFTLEIILKMTAYGAFLHKGSFCRNYFNILDLLVVSVSLISFGIQSSAINVVKILRVLRVLRPLRAINRAKGLKHVVQCVFVAIRTIGNIVIVTTLLQFMFACIGVQLFKGKLYTCSDSSKQTEAECKGNYITYKDGEVDHPIIQPRSWENSKFDFDNVLAAMMALFTVSTFEGWPELLYRSIDSHTEDKGPIYNYRVEISIFFIIYIIIIAFFMMNIFVGFVIVTFQEQGEQEYKNCELDKNQRQCVEYALKARPLRRYIPKNQHQYKVWYVVNSTYFEYLMFVLILLNTICLAMQHYGQSCLFKIAMNILNMLFTGLFTVEMILKLIAFKPKGYFSDPWNVFDFLIVIGSIIDVILSETNHYFCDAWNTFDALIVVGSIVDIAITEVNPAEHTQCSPSMNAEENSRISITFFRLFRVMRLVKLLSRGEGIRTLLWTFIKSFQALPYVALLIVMLFFIYAVIGMQVFGKIALNDTTEINRNNNFQTFPQAVLLLFRCATGEAWQDIMLACMPGKKCAPESEPSNSTEGETPCGSSFAVFYFISFYMLCAFLIINLFVAVIMDNFDYLTRDWSILGPHHLDEFKRIWAEYDPEAKGRIKHLDVVTLLRRIQPPLGFGKLCPHRVACKRLVSMNMPLNSDGTVMFNATLFALVRTALRIKTEGNLEQANEELRAIIKKIWKRTSMKLLDQVVPPAGDDEVTVGKFYATFLIQEYFRKFKKRKEQGLVGKPSQRNALSLQAGLRTLHDIGPEIRRAISGDLTAEEELDKAMKEAVSAASEDDIFRRAGGLFGNHVSYYQSDGRSAFPQTFTTQRPLHINKAGSSQGDTESPSHEKLVDSTFTPSSYSSTGSNANINNANNTALGRLPRPAGYPSTVSTVEGHGPPLSPAIRVQEVAWKLSSNRERHVPMCEDLELRRDSGSAGTQAHCLLLRKANPSRCHSRESQAAMAGQEETSQDETYEVKMNHDTEACSEPSLLSTEMLSYQDDENRQLTLPEEDKRDIRQSPKRGFLRSASLGRRASFHLECLKRQKDRGGDISQKTVLPLHLVHHQALAVAGLSPLLQRSHSPASFPRPFATPPATPGSRGWPPQPVPTLRLEGVESSEKLNSSFPSIHCGSWAETTPGGGGSSAARRVRPVSLMVPSQAGAPGRQFHGSASSLVEAVLISEGLGQFAQDPKFIEVTTQELADACDMTIEEMESAADNILSGGAPQSPNGALLPFVNCRDAGQDRAGGEEDAGCVRARGRPSEEELQDSRVYVSSL
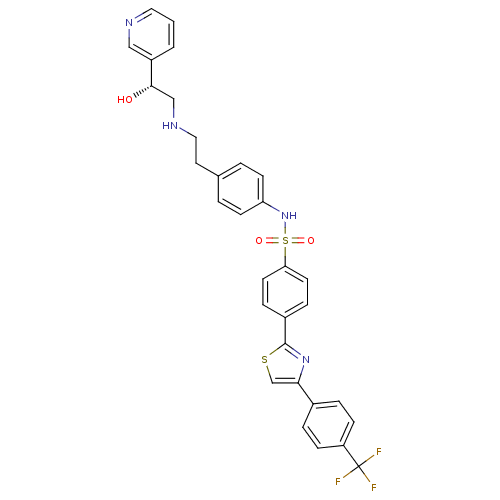
Inhibitor
Name:
BDBM50092645
Synonyms:
(R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)ethyl)phenyl)-4-(4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)benzenesulfonamide | CHEMBL111201 | L-796568 | N-{4-[2-((R)-2-Hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[4-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide | N-{4-[2-((R)-2-hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[3-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide | N-{4-[2-(2-Hydroxy-2-pyridin-3-yl-ethylamino)-ethyl]-phenyl}-4-[4-(4-trifluoromethyl-phenyl)-thiazol-2-yl]-benzenesulfonamide
Type:
Small organic molecule
Emp. Form.:
C31H27F3N4O3S2
Mol. Mass.:
624.696
SMILES:
O[C@@H](CNCCc1ccc(NS(=O)(=O)c2ccc(cc2)-c2nc(cs2)-c2ccc(cc2)C(F)(F)F)cc1)c1cccnc1