Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM28802
Substrate
n/a
Meas. Tech.
ChEMBL_1589971 (CHEMBL3831060)
IC50
>150000±n/a nM
Citation
 Shi, Y; Li, J; Kennedy, LJ; Tao, S; Hernández, AS; Lai, Z; Chen, S; Wong, H; Zhu, J; Trehan, A; Lim, NK; Zhang, H; Chen, BC; Locke, KT; O'Malley, KM; Zhang, L; Srivastava, RA; Miao, B; Meyers, DS; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Harrity, T; Kunselman, LK; Cap, M; Muckelbauer, J; Chang, C; Krystek, SR; Li, YX; Hosagrahara, V; Zhang, L; Kadiyala, P; Xu, C; Blanar, MA; Zahler, R; Mukherjee, R; Cheng, PT; Tino, JA Discovery and Preclinical Evaluation of BMS-711939, an Oxybenzylglycine Based PPARa Selective Agonist. ACS Med Chem Lett 7:590-4 (2016) [PubMed] Article
Shi, Y; Li, J; Kennedy, LJ; Tao, S; Hernández, AS; Lai, Z; Chen, S; Wong, H; Zhu, J; Trehan, A; Lim, NK; Zhang, H; Chen, BC; Locke, KT; O'Malley, KM; Zhang, L; Srivastava, RA; Miao, B; Meyers, DS; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Harrity, T; Kunselman, LK; Cap, M; Muckelbauer, J; Chang, C; Krystek, SR; Li, YX; Hosagrahara, V; Zhang, L; Kadiyala, P; Xu, C; Blanar, MA; Zahler, R; Mukherjee, R; Cheng, PT; Tino, JA Discovery and Preclinical Evaluation of BMS-711939, an Oxybenzylglycine Based PPARa Selective Agonist. ACS Med Chem Lett 7:590-4 (2016) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
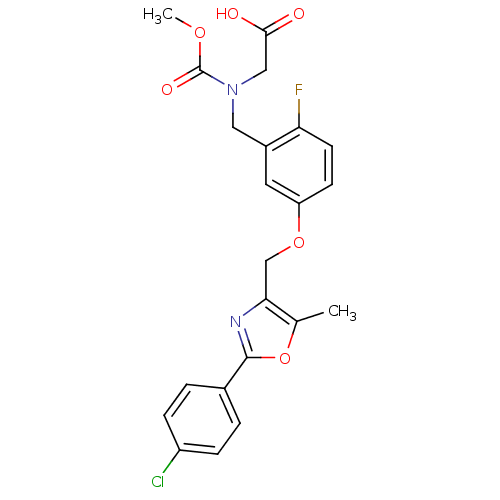
Inhibitor
Name:
BDBM28802
Synonyms:
2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}-2-fluorophenyl)methyl](methoxycarbonyl)amino}acetic acid | BMS-711939
Type:
Small organic molecule
Emp. Form.:
C22H20ClFN2O6
Mol. Mass.:
462.855
SMILES:
COC(=O)N(CC(O)=O)Cc1cc(OCc2nc(oc2C)-c2ccc(Cl)cc2)ccc1F