Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2B
Ligand
BDBM50163020
Substrate
n/a
Meas. Tech.
ChEMBL_1621594 (CHEMBL3863877)
IC50
3260±n/a nM
Citation
 Tosh, DK; Ciancetta, A; Warnick, E; Crane, S; Gao, ZG; Jacobson, KA Structure-Based Scaffold Repurposing for G Protein-Coupled Receptors: Transformation of Adenosine Derivatives into 5HT J Med Chem 59:11006-11026 (2016) [PubMed] Article
Tosh, DK; Ciancetta, A; Warnick, E; Crane, S; Gao, ZG; Jacobson, KA Structure-Based Scaffold Repurposing for G Protein-Coupled Receptors: Transformation of Adenosine Derivatives into 5HT J Med Chem 59:11006-11026 (2016) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2B
Synonyms:
5-HT-2B | 5-HT2B | 5-hydroxytryptamine (serotonin) receptor 2B [Homo sapiens] | 5-hydroxytryptamine receptor 2B (5-HT2B) | 5-hydroxytryptamine receptor 2C (5HT2C) | 5HT2B_HUMAN | HTR2B | Serotonin (5-HT3) receptor | Serotonin 2b (5-HT2b) receptor | Serotonin Receptor 2B
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
54312.47
Organism:
Homo sapiens (Human)
Description:
Receptor binding assays were performed using human clone stably expressed in CHO cells.
Residue:
481
Sequence:
MALSYRVSELQSTIPEHILQSTFVHVISSNWSGLQTESIPEEMKQIVEEQGNKLHWAALLILMVIIPTIGGNTLVILAVSLEKKLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEAMWPLPLVLCPAWLFLDVLFSTASIMHLCAISVDRYIAIKKPIQANQYNSRATAFIKITVVWLISIGIAIPVPIKGIETDVDNPNNITCVLTKERFGDFMLFGSLAAFFTPLAIMIVTYFLTIHALQKKAYLVKNKPPQRLTWLTVSTVFQRDETPCSSPEKVAMLDGSRKDKALPNSGDETLMRRTSTIGKKSVQTISNEQRASKVLGIVFFLFLLMWCPFFITNITLVLCDSCNQTTLQMLLEIFVWIGYVSSGVNPLVYTLFNKTFRDAFGRYITCNYRATKSVKTLRKRSSKIYFRNPMAENSKFFKKHGIRNGINPAMYQSPMRLRSSTIQSSSIILLDTLLLTENEGDKTEEQVSYV
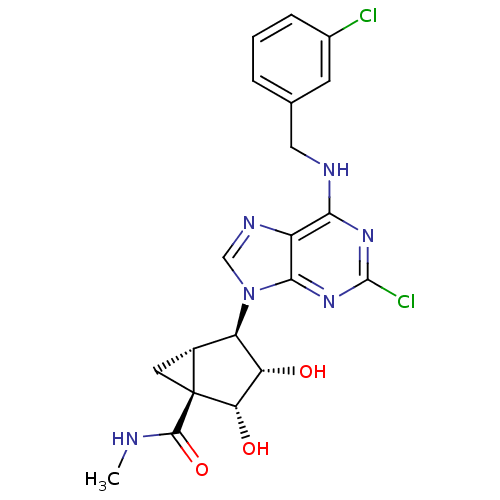
Inhibitor
Name:
BDBM50163020
Synonyms:
(1S,2R,3S,4R,5S)-4-(6-(3-chlorobenzylamino)-2-chloro-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide | (1S,2R,3S,4R,5S)-4-[2-Chloro-6-(3-chloro-benzylamino)-purin-9-yl]-2,3-dihydroxy-bicyclo[3.1.0]hexane-1-carboxylic acid methylamide | 1N-methyl-4-[2-chloro-6-(3-chlorobenzylamino)-9H-9-purinyl]-2,3-dihydroxy-(1S,2R,3S,4R,5S)-bicyclo[3.1.0]hexane-1-carboxamide | CHEMBL175543
Type:
Small organic molecule
Emp. Form.:
C20H20Cl2N6O3
Mol. Mass.:
463.317
SMILES:
CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r|