Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM50207108
Substrate
n/a
Meas. Tech.
ChEMBL_1630051 (CHEMBL3872757)
Ki
1.1±n/a nM
Citation
More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A
Type:
n/a
Mol. Mass.:
46122.49
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
422
Sequence:
MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADTRHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGNSKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRQ
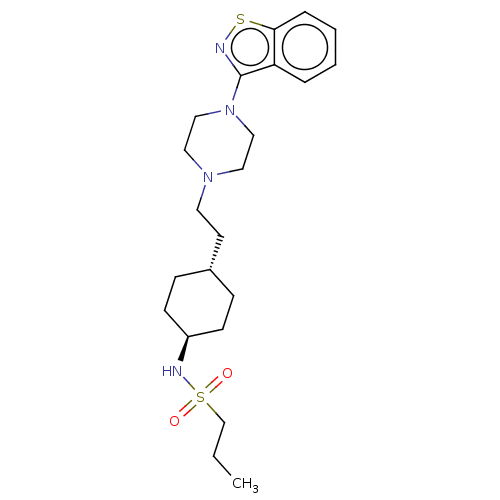
Inhibitor
Name:
BDBM50207108
Synonyms:
CHEMBL3920432
Type:
Small organic molecule
Emp. Form.:
C22H34N4O2S2
Mol. Mass.:
450.661
SMILES:
CCCS(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:10.10,wD:7.6,(41.02,-21.07,;39.48,-21.06,;38.71,-22.4,;37.17,-22.39,;37.16,-23.93,;35.83,-23.16,;36.4,-21.06,;34.86,-21.06,;34.09,-19.73,;32.55,-19.74,;31.79,-21.07,;30.25,-21.07,;29.47,-19.74,;27.93,-19.74,;27.16,-18.41,;25.62,-18.41,;24.86,-19.74,;25.63,-21.07,;27.17,-21.07,;23.32,-19.74,;22.42,-20.99,;20.95,-20.51,;20.95,-18.97,;19.8,-17.94,;20.13,-16.44,;21.59,-15.96,;22.73,-16.99,;22.41,-18.49,;32.55,-22.4,;34.09,-22.4,)|