Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM50005118
Substrate
n/a
Ki
4000±n/a nM
Comments
PDSP_1584
Citation
 Seeman, P; Tallerico, T Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry 3:123-34 (1998) [PubMed] Article
Seeman, P; Tallerico, T Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry 3:123-34 (1998) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2A | 5-HT2 | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_RAT | Htr2 | Htr2a | Serotonin Receptor 2A
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
52852.05
Organism:
Rattus norvegicus (rat)
Description:
Rat cortex membranes 5-HT2A receptors.
Residue:
471
Sequence:
MEILCEDNISLSSIPNSLMQLGDGPRLYHNDFNSRDANTSEASNWTIDAENRTNLSCEGYLPPTCLSILHLQEKNWSALLTTVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAIWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVAFFIPLTIMVITYFLTIKSLQKEATLCVSDLSTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYAGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNENVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENRKPLQLILVNTIPALAYKSSQLQVGQKKNSQEDAEQTVDDCSMVTLGKQQSEENCTDNIETVNEKVSCV
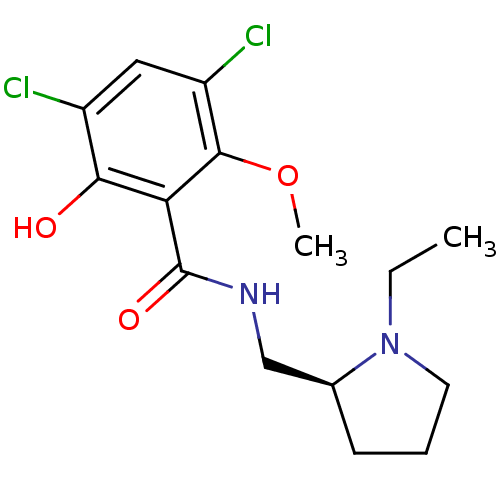
Inhibitor
Name:
BDBM50005118
Synonyms:
(S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide | (S)-3,5-dichloro-N-((1-ethylpyrrolidin-2-yl)methyl)-2-hydroxy-6-methoxybenzamide | (S)3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide | 3,5-Dichloro-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide | 3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide | 3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide (raclopride) | 3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide(Raclopride) | CHEMBL8809 | RACLOPRIDE | Raclopride;3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxy-benzamide
Type:
Small organic molecule
Emp. Form.:
C15H20Cl2N2O3
Mol. Mass.:
347.237
SMILES:
CCN1CCC[C@H]1CNC(=O)c1c(O)c(Cl)cc(Cl)c1OC