Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM50167898
Substrate
n/a
Ki
>10000±n/a nM
Comments
PDSP_1676
Citation
 Reavill, C; Taylor, SG; Wood, MD; Ashmeade, T; Austin, NE; Avenell, KY; Boyfield, I; Branch, CL; Cilia, J; Coldwell, MC; Hadley, MS; Hunter, AJ; Jeffrey, P; Jewitt, F; Johnson, CN; Jones, DN; Medhurst, AD; Middlemiss, DN; Nash, DJ; Riley, GJ; Routledge, C; Stemp, G; Thewlis, KM; Trail, B; Vong, AK; Hagan, JJ Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294:1154-65 (2000) [PubMed]
Reavill, C; Taylor, SG; Wood, MD; Ashmeade, T; Austin, NE; Avenell, KY; Boyfield, I; Branch, CL; Cilia, J; Coldwell, MC; Hadley, MS; Hunter, AJ; Jeffrey, P; Jewitt, F; Johnson, CN; Jones, DN; Medhurst, AD; Middlemiss, DN; Nash, DJ; Riley, GJ; Routledge, C; Stemp, G; Thewlis, KM; Trail, B; Vong, AK; Hagan, JJ Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294:1154-65 (2000) [PubMed] More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A
Type:
undefined
Mol. Mass.:
52607.65
Organism:
Homo sapiens (Human)
Description:
P28223
Residue:
471
Sequence:
MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGCLSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKSSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
Inhibitor
Name:
BDBM50167898
Synonyms:
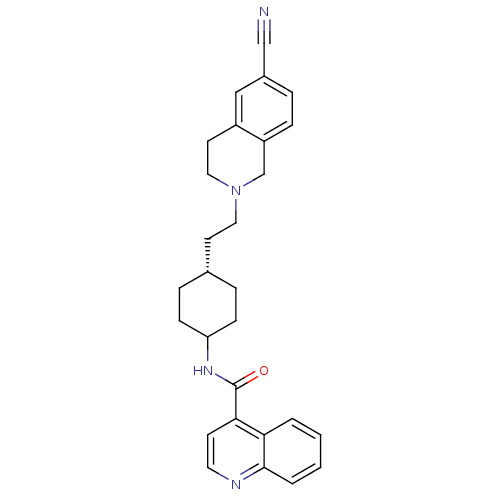
CHEMBL85606 | N-((1r,4r)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide | N-(-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide | Quinoline-4-carboxylic acid {4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-cyclohexyl}-amide | SB-277011 | SB-277011-A | trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)-ethyl]cyclo-hexyl]-4-quinolinecarboxamide | trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)ethyl]-cyclohexyl]-4-quinolininecarboxamide
Type:
Small organic molecule
Emp. Form.:
C28H30N4O
Mol. Mass.:
438.564
SMILES:
O=C(NC1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |wD:6.6,(-3.87,-1.76,;-3.86,-3.3,;-2.52,-4.08,;-1.18,-3.3,;-1.19,-1.76,;.15,-.99,;1.48,-1.76,;2.83,-.99,;4.16,-1.76,;5.49,-.99,;5.49,.54,;6.84,1.31,;8.17,.53,;9.5,1.3,;10.83,.53,;10.81,-1.01,;9.48,-1.78,;8.16,-1.01,;6.84,-1.78,;12.17,1.29,;13.5,2.07,;1.48,-3.3,;.15,-4.08,;-5.19,-4.09,;-5.19,-5.64,;-6.54,-6.41,;-7.87,-5.64,;-7.87,-4.09,;-9.19,-3.33,;-9.2,-1.81,;-7.87,-1.03,;-6.54,-1.78,;-6.54,-3.33,)|