Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA primase
Ligand
BDBM50336799
Substrate
n/a
Meas. Tech.
Quatitative Inhibition Analysis Assay
pH
8.8±n/a
IC50
8.2e+3± 1.3e+3 nM
Comments
extracted
Citation
 Biswas, T; Green, KD; Garneau-Tsodikova, S; Tsodikov, OV Discovery of inhibitors of Bacillus anthracis primase DnaG. Biochemistry 52:6905-10 (2013) [PubMed] Article
Biswas, T; Green, KD; Garneau-Tsodikova, S; Tsodikov, OV Discovery of inhibitors of Bacillus anthracis primase DnaG. Biochemistry 52:6905-10 (2013) [PubMed] Article More Info.:
Target
Name:
DNA primase
Synonyms:
DNA primase (Ba DnaG)
Type:
Protein
Mol. Mass.:
68258.37
Organism:
Bacillus anthracis (Firmicutes)
Description:
A0A6L8PXB3
Residue:
598
Sequence:
MGNRIPEEVVEQIRTSSDIVEVIGEYVQLRKQGRNYFGLCPFHGENSPSFSVSSDKQIFHCFGCGEGGNVFSFLMKMEGLAFTEAVQKLGERNGIAVAEYTSGQGQQEDISDDTVIMQQAHELLKKYYHHLLVNTEEGNEALSYLLKRGITKEMIEKFEIGYASPAWDAATKILQKRGLSLSSMEQAGLLIRSEKDGSHYDRFRGRVMFPIYTLQGKVIAFSGRALGDDTPKYLNSPETPIFHKSKLLYNFHQARPFIRKRGQVVLFEGYADVLAAVKSGVEEAVATMGTALTEEQAKLLRRNVETVVLCYDGDKAGREATMKAGQLLLQVGCQVKVTSLPDKLDPDEYVQQYGTTAFENLVKSSISFVGFKINYLRLGKNLQDESGKEEYVKSVLKELSLLQDAMQAESYLKSLSQEFSYSMETLLNQLHQYRKEQKVQQKQVKQVSKPSQIVQTKPKLTGFERAEREIIYHMLQSPEVAVRMESHIEDFHTEEHKGILYELYAYYEKGNEPSVGTFLSWLSDEKLKNIITDISTDEFINPEYTEEVLQSHLETLRRHQEKLEKMEIIFKIKQMEKTDPVEAAKYYVAYLQNQKARK
Inhibitor
Name:
BDBM50336799
Synonyms:
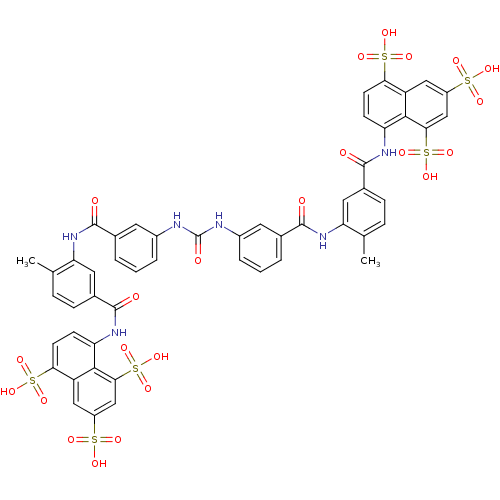
5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino)]tris[1,3-benzenesulfonate analogue | 8,8'-(Carbonylbis(imino-3,1-phenylenecarbonylimino(4-methyl-3,1-phenylene)carbonylimino))bisnaphthalene-1,3,5-trisulphonic acid | 8,8'-[CARBONYLBIS[IMINO-3,1-PHENYLENECARBONYLIMINO(4-METHYL-3,1-PHENYLENE)CARBONYLIMINO]]BIS-1,3,5-NAPHTHALENETRISULFONIC ACID | 8,8'-[Carbonylbis[imino-3,1-phenylenecarbonylimino(4-methyl-3,1-phenylene)carbonyl-imino]]bis-1,3,5-naphthalenetrisulfonic acid(suramin) | 8-[(4-methyl-3-{[3-({[3-({2-methyl-5-[(4,6,8-trisulfonaphthalen-1-yl)carbamoyl]phenyl}carbamoyl)phenyl]carbamoyl}amino)benzene]amido}benzene)amido]naphthalene-1,3,5-trisulfonic acid | CHEMBL265502 | Germanin | Suramin hexasodium | US8835659, Suramin | Urea derivative | suramin
Type:
Small organic molecule
Emp. Form.:
C51H40N6O23S6
Mol. Mass.:
1297.28
SMILES:
Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O