Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cathepsin K
Ligand
BDBM138494
Substrate
n/a
Meas. Tech.
Inhibition Assay
pH
3.5±n/a
Temperature
298.15±n/a K
IC50
6300±n/a nM
Comments
extracted
Citation
 Anderskewitz, R; Grauert, M; Grundl, M; Oost, T; Pautsch, A; Peters, S Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C US Patent US8877775 Publication Date 11/4/2014
Anderskewitz, R; Grauert, M; Grundl, M; Oost, T; Pautsch, A; Peters, S Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C US Patent US8877775 Publication Date 11/4/2014 More Info.:
Target
Name:
Cathepsin K
Synonyms:
CATK_HUMAN | CTSK | CTSO | CTSO2 | Cathepsin O | Cathepsin O2 | Cathepsin X
Type:
Enzyme
Mol. Mass.:
36975.68
Organism:
Homo sapiens (Human)
Description:
P43235
Residue:
329
Sequence:
MWGLKVLLLPVVSFALYPEEILDTHWELWKKTHRKQYNNKVDEISRRLIWEKNLKYISIHNLEASLGVHTYELAMNHLGDMTSEEVVQKMTGLKVPLSHSRSNDTLYIPEWEGRAPDSVDYRKKGYVTPVKNQGQCGSCWAFSSVGALEGQLKKKTGKLLNLSPQNLVDCVSENDGCGGGYMTNAFQYVQKNRGIDSEDAYPYVGQEESCMYNPTGKAAKCRGYREIPEGNEKALKRAVARVGPVSVAIDASLTSFQFYSKGVYYDESCNSDNLNHAVLAVGYGIQKGNKHWIIKNSWGENWGNKGYILMARNKNNACGIANLASFPKM
Inhibitor
Name:
BDBM138494
Synonyms:
US8877775, 4 | US9073869, 4
Type:
Small organic molecule
Emp. Form.:
C26H28N4O2
Mol. Mass.:
428.5261
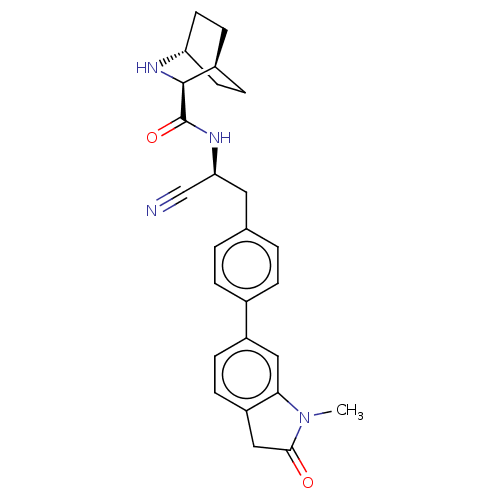
SMILES:
CN1C(=O)Cc2ccc(cc12)-c1ccc(C[C@H](NC(=O)[C@H]2N[C@H]3CC[C@@H]2CC3)C#N)cc1 |r,wU:20.21,25.26,wD:16.18,22.23,TLB:18:20:27.26:24.23,(7.91,-1,;7.14,-2.34,;8.04,-3.58,;9.58,-3.58,;7.14,-4.83,;5.67,-4.35,;4.34,-5.12,;3.01,-4.35,;3.01,-2.81,;4.34,-2.04,;5.67,-2.81,;1.67,-2.04,;.34,-2.81,;-.99,-2.04,;-.99,-.5,;-2.33,.27,;-2.33,1.81,;-3.66,2.58,;-5,1.81,;-5,.27,;-6.33,2.58,;-7.87,2.59,;-8.04,4.03,;-6.33,5.07,;-4.9,4.92,;-6.37,4.03,;-7.83,5.06,;-9.28,5.12,;-.99,2.58,;.34,3.35,;.34,.27,;1.67,-.5,)|