Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nitric oxide synthase, inducible
Ligand
BDBM50330873
Substrate
n/a
Meas. Tech.
Enzyme Inhibition Assay
Ki
18200±n/a nM
Citation
 Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015
Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 Publication Date 7/28/2015 More Info.:
Target
Name:
Nitric oxide synthase, inducible
Synonyms:
HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS
Type:
Homodimer
Mol. Mass.:
131141.95
Organism:
Homo sapiens (Human)
Description:
P35228
Residue:
1153
Sequence:
MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPLVETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIMTPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQLTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNIRSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYGRFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVGGLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINIAVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEMLNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVTILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPGNGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGDELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDLSKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQPALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQLLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQLPILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCFVRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPDEDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLYVCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDRVAVQPSSLEMSAL
Inhibitor
Name:
BDBM50330873
Synonyms:
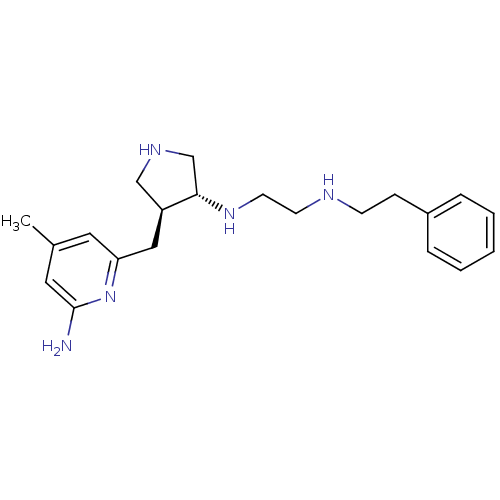
CHEMBL1277786 | US9090589, 4 | trans rac-N1-(4-((6-amino-4-methylpyridin-2-yl)methyl)pyrrolidin-3-yl)-N2-phenethylethane-1,2-diamine
Type:
Small organic molecule
Emp. Form.:
C21H31N5
Mol. Mass.:
353.5043
SMILES:
Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2ccccc2)c1 |r|