Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Muscarinic acetylcholine receptor M3
Ligand
BDBM221902
Substrate
n/a
Meas. Tech.
Muscarinic Receptor Binding Assay
IC50
1.6±n/a nM
Citation
 Prat Quinones, M; Fonquerna Pou, S; Puig Duran, C; Lumeras Amador, W; Aiguade Bosch, J; Caturla Jovaloyes, JF Cyclohexylamine derivatives having β2 adrenergic agonist and M3 muscarinic antagonist activities US Patent US9315463 Publication Date 4/19/2016
Prat Quinones, M; Fonquerna Pou, S; Puig Duran, C; Lumeras Amador, W; Aiguade Bosch, J; Caturla Jovaloyes, JF Cyclohexylamine derivatives having β2 adrenergic agonist and M3 muscarinic antagonist activities US Patent US9315463 Publication Date 4/19/2016 More Info.:
Target
Name:
Muscarinic acetylcholine receptor M3
Synonyms:
ACM3_HUMAN | CHRM3 | Cholinergic, muscarinic M3 | Muscarinic Receptors M3 | Muscarinic receptor M3 | RecName: Full=Muscarinic acetylcholine receptor M3
Type:
Enzyme
Mol. Mass.:
66151.03
Organism:
Homo sapiens (Human)
Description:
P20309
Residue:
590
Sequence:
MTLHNNSTTSPLFPNISSSWIHSPSDAGLPPGTVTHFGSYNVSRAAGNFSSPDGTTDDPLGGHTVWQVVFIAFLTGILALVTIIGNILVIVSFKVNKQLKTVNNYFLLSLACADLIIGVISMNLFTTYIIMNRWALGNLACDLWLAIDYVASNASVMNLLVISFDRYFSITRPLTYRAKRTTKRAGVMIGLAWVISFVLWAPAILFWQYFVGKRTVPPGECFIQFLSEPTITFGTAIAAFYMPVTIMTILYWRIYKETEKRTKELAGLQASGTEAETENFVHPTGSSRSCSSYELQQQSMKRSNRRKYGRCHFWFTTKSWKPSSEQMDQDHSSSDSWNNNDAAASLENSASSDEEDIGSETRAIYSIVLKLPGHSTILNSTKLPSSDNLQVPEEELGMVDLERKADKLQAQKSVDDGGSFPKSFSKLPIQLESAVDTAKTSDVNSSVGKSTATLPLSFKEATLAKRFALKTRSQITKRKRMSLVKEKKAAQTLSAILLAFIITWTPYNIMVLVNTFCDSCIPKTFWNLGYWLCYINSTVNPVCYALCNKTFRTTFKMLLLCQCDKKKRRKQQYQQRQSVIFHKRAPEQAL
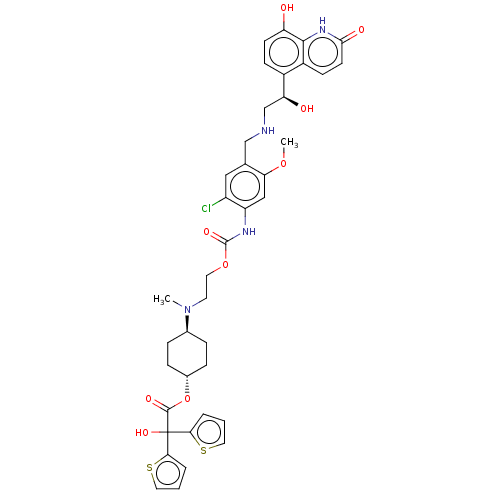
Inhibitor
Name:
BDBM221902
Synonyms:
US9315463, 12
Type:
Small organic molecule
Emp. Form.:
C39H43ClN4O9S2
Mol. Mass.:
811.363
SMILES:
COc1cc(NC(=O)OCCN(C)[C@H]2CC[C@@H](CC2)OC(=O)C(O)(c2cccs2)c2cccs2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:41.45,13.12,wD:16.19,(-5.85,-3.32,;-4.52,-2.55,;-4.52,-1.01,;-3.19,-.24,;-3.19,1.3,;-1.85,2.07,;-.52,1.3,;-.52,-.24,;.82,2.07,;2.15,1.3,;3.48,2.07,;4.82,1.3,;4.82,-.24,;6.15,2.07,;6.15,3.61,;7.48,4.38,;8.82,3.61,;8.82,2.07,;7.48,1.3,;10.15,4.38,;11.49,3.61,;11.49,2.07,;12.82,4.38,;12.05,5.72,;13.59,3.05,;15.13,3.05,;15.6,1.58,;14.36,.68,;13.11,1.58,;14.15,5.15,;14.15,6.69,;15.62,7.17,;16.52,5.92,;15.62,4.68,;-4.52,2.07,;-4.52,3.61,;-5.85,1.3,;-5.85,-.24,;-7.19,-1.01,;-8.52,-.24,;-9.85,-1.01,;-11.19,-.24,;-11.19,1.3,;-12.52,-1.01,;-13.85,-.24,;-15.19,-1.01,;-15.19,-2.55,;-16.52,-3.32,;-13.85,-3.32,;-13.85,-4.86,;-12.52,-5.63,;-12.52,-7.17,;-11.19,-4.86,;-11.19,-3.32,;-12.52,-2.55,)|