Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Protein arginine N-methyltransferase 1
Ligand
BDBM502101
Substrate
n/a
Meas. Tech.
PRMT1 Enzymatic Assay
IC50
46.0±n/a nM
Citation
 Di Francesco, ME; Jones, P; McAfoos, TJ Ethanediamine-heterocycle derivatives as inhibitors of protein arginine methyltransferases US Patent US11028083 Publication Date 6/8/2021
Di Francesco, ME; Jones, P; McAfoos, TJ Ethanediamine-heterocycle derivatives as inhibitors of protein arginine methyltransferases US Patent US11028083 Publication Date 6/8/2021 More Info.:
Target
Name:
Protein arginine N-methyltransferase 1
Synonyms:
2.1.1.319 | ANM1_HUMAN | HMT2 | HRMT1L2 | HRMT1L2 {ECO:0000303|PubMed:11097842 | Histone-arginine N-methyltransferase PRMT1 | IR1B4 | Interferon receptor 1-bound protein 4 | PRMT1 | Protein arginine N-methyltransferase 1 | Protein-arginine N-methyltransferase 1 | Synonyms=HMT2
Type:
PROTEIN
Mol. Mass.:
42451.61
Organism:
Homo sapiens
Description:
ChEMBL_100878
Residue:
371
Sequence:
MAAAEAANCIMENFVATLANGMSLQPPLEEVSCGQAESSEKPNAEDMTSKDYYFDSYAHFGIHEEMLKDEVRTLTYRNSMFHNRHLFKDKVVLDVGSGTGILCMFAAKAGARKVIGIECSSISDYAVKIVKANKLDHVVTIIKGKVEEVELPVEKVDIIISEWMGYCLFYESMLNTVLYARDKWLAPDGLIFPDRATLYVTAIEDRQYKDYKIHWWENVYGFDMSCIKDVAIKEPLVDVVDPKQLVTNACLIKEVDIYTVKVEDLTFTSPFCLQVKRNDYVHALVAYFNIEFTRCHKRTGFSTSPESPYTHWKQTVFYMEDYLTVKTGEEIFGTIGMRPNAKNNRDLDFTIDLDFKGQLCELSCSTDYRMR
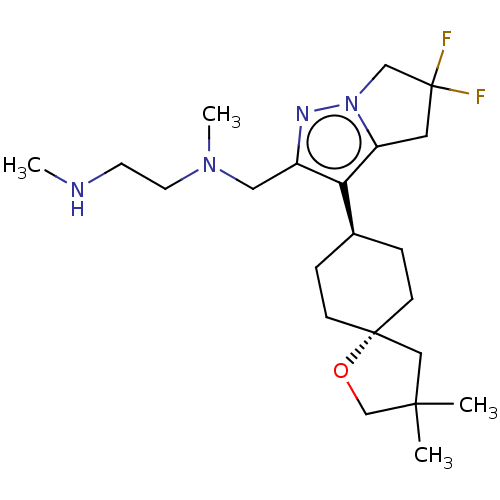
Inhibitor
Name:
BDBM502101
Synonyms:
US11028083, Example 78b
Type:
Small organic molecule
Emp. Form.:
C22H36F2N4O
Mol. Mass.:
410.5442
SMILES:
CNCCN(C)Cc1nn2CC(F)(F)Cc2c1[C@H]1CC[C@@]2(CC(C)(C)CO2)CC1 |r,wU:20.28,wD:17.18,(8.05,7.38,;6.71,8.15,;5.38,7.38,;4.05,8.15,;2.71,7.38,;2.71,5.84,;1.38,8.15,;.05,7.38,;.6,5.95,;-.6,4.98,;-1,3.49,;-2.54,3.41,;-2.54,1.87,;-3.87,2.64,;-3.09,4.85,;-1.89,5.81,;-1.49,7.3,;-2.83,8.07,;-2.83,9.61,;-4.16,10.38,;-5.49,9.61,;-5.49,11.15,;-6.96,11.63,;-8.05,12.72,;-6.56,13.12,;-7.86,10.38,;-6.96,9.14,;-5.49,8.07,;-4.16,7.3,)|