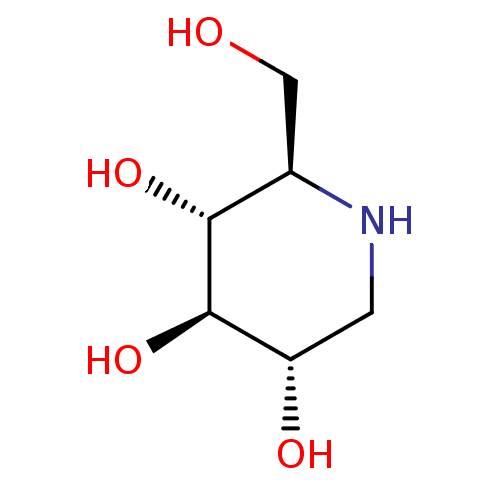
BDBM18351 (2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-triol, 10::(2R,3R,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-triol::1-Deoxynojirimycin::1-deoxynojirimycin (DNJ)::CHEMBL307429::US20230339856, Compound DNJ::US9181184, 1::dNM
SMILES C1[C@@H]([C@H]([C@@H]([C@H](N1)CO)O)O)O
InChI Key InChIKey=LXBIFEVIBLOUGU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 134 hits for monomerid = 18351
Found 134 hits for monomerid = 18351
Affinity DataKi: 14nMpH: 6.0Assay Description:Competitive Inhibition constant on rice alpha Glucosidase at pH 6.0More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Tested in vitro for the inhibition constant against rat small intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration against rice alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibitory concentration against human alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibitory activity against alpha-Glucosidase from riceMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me...More data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Binding affinity to human lysosomal acid alpha-glucosidase assessed as inhibition constant incubated for 30 mins by fluorescence based spectrophotome...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of maltase in human Caco-2 cell model system after 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of rat intestinal brush border membrane maltaseMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Tested in vitro for the inhibition constant against human lysosomal alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 160nMpH: 5.0Assay Description:Competitive Inhibition constant on rice alpha Glucosidase at pH 5.0More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of rat intestinal brush border membrane isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of rabbit muscle amylo-1,6-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of rat intestinal sucrase using sucrose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibitory activity against rat intestinal sucrase using disaccharideMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of rat intestinal brush border membrane sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
TargetTrehalose synthase/amylase TreS(Mycobacterium tuberculosis (strain CDC 1551 / Oshk...)
University of British Columbia
University of British Columbia
Affinity DataKi: 250nMAssay Description:The inhibition of TreS by a range of known alpha-glucosidase inhibitor was assayed.More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of Glycosidases (isomaltase)in rat intestinal brush border membranes by D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of rat intestinal isomaltase using isomaltase as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibitory activity against rat intestinal isomaltase using disaccharideMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of Glycosidases (maltase) in rat intestinal brush border membranes by D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibitory activity against rat intestinal maltase using disaccharideMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of rat intestinal maltase using moltose as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of rat intestinal maltase assessed as D-glucose release after 30 mins by Glucose B-testMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of rat liver lysosome alpha-glucosidase assessed as D-glucose release after 30 mins by Glucose B-testMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:The rhGAA enzyme sold under the name Myozyme used comes from residues of perfusions of the recombinant enzyme used for treating, by enzyme therapy, t...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of rat intestinal sucrase assessed as production of p-nitrophenol at by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass...More data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Inhibition of rat intestinal isomaltase assessed as production of p-nitrophenol at by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Inhibitory concentration against rat intestinal maltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMpH: 4.5 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human lysosomal alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Tested for competitive inhibition of endoplasmic reticulum alpha-glucosidase II.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human lysosomal alpha glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of lysosomal alpha-glucosidase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.67E+3nMAssay Description:Inhibition of alpha glucosidase from bacillus stearothermophilusMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of maltase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of sucrase by HPLCMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:The inhibiting activity on recombinant human acid α-glucosidase (rhGAA) of the compounds is implemented using the Fluopol-ABPP method (Fluoresce...More data for this Ligand-Target Pair
Affinity DataIC50: 8.03E+3nMAssay Description:Inhibition of alpha-glucosidase (unknown origin) preincubated for 5 mins followed by addition of pNPG substrate and measured after 30 mins by spectro...More data for this Ligand-Target Pair
Affinity DataKi: 9.50E+3nMAssay Description:Inhibitory activity against sweet almond Beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of glycogen glycogen de-branching enzyme by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of equine serum BuChE using p-nitrophenyl glycopyranoside as substrate by DTNB-reagent based Ellman's methodMore data for this Ligand-Target Pair
TargetKiller cell lectin-like receptor subfamily B member 1A(Rat)
University of Pisa
Curated by ChEMBL
University of Pisa
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Activation of rat NKR-P1AMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of Agrobacterium sp. beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of Agrobacterium sp. beta glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Compound tested for inhibition of alpha-galactosidase from Aspergillus nigerMore data for this Ligand-Target Pair