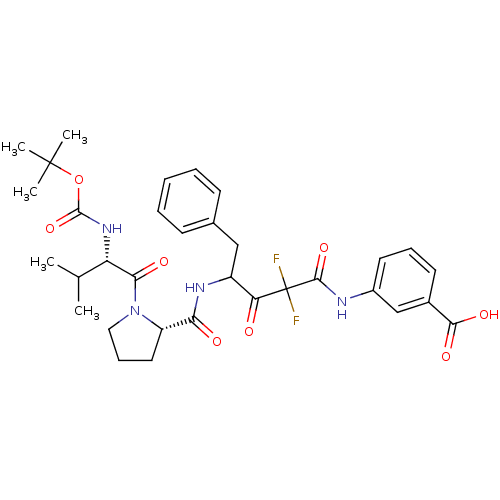
BDBM50068894 3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methyl-butyryl)-pyrrolidine-2-carbonyl]-amino}-2,2-difluoro-3-oxo-5-phenyl-pentanoylamino)-benzoic acid::3-(4-{[1-(2-tert-Butoxycarbonylamino-3-methyl-butyryl)-pyrrolidine-2-carbonyl]-amino}-2,2-difluoro-3-oxo-5-phenyl-pentanoylamino)-benzoic acid::CHEMBL287318
SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(Cc1ccccc1)C(=O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O
InChI Key InChIKey=DWKQSWOPFOFIHM-DJHGOXGWSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50068894
Found 4 hits for monomerid = 50068894
Affinity DataKi: 5.60nMAssay Description:Compound was evaluated for inhibitory activity against human heart chymase (HHC)More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:In vitro inhibitory activity was determined against human heart chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 364nMAssay Description:In vitro inhibitory activity was determined against bovine pancreas chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 364nMAssay Description:Compound was evaluated for inhibitory activity against ChymotrypsinogenMore data for this Ligand-Target Pair