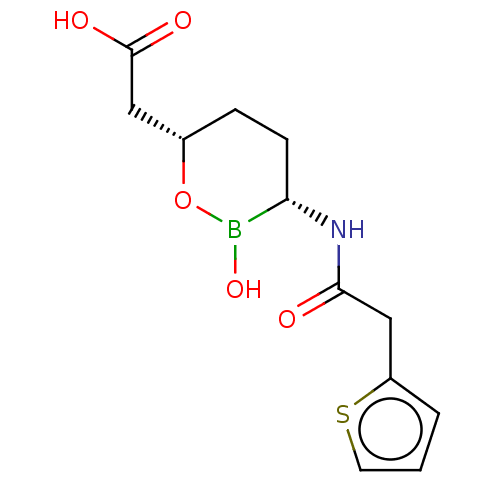
BDBM50089084 CHEMBL3317857::Vaborbactam
SMILES OB1O[C@H](CC(O)=O)CC[C@@H]1NC(=O)Cc1cccs1
InChI Key InChIKey=IOOWNWLVCOUUEX-WPRPVWTQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50089084
Found 12 hits for monomerid = 50089084
Affinity DataKi: 56nMAssay Description:Inhibition of recombinant Escherichia coli KPC-2 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition and measured e...More data for this Ligand-Target Pair
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of recombinant Escherichia coli VIM-1 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition measured every...More data for this Ligand-Target Pair
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Acinetobacter baumannii OXA-23 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition measured every 10 ...More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Rempex Pharmaceuticals
Curated by ChEMBL
Rempex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of urokinase (unknown origin) using NGK-pNA as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by s...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Rempex Pharmaceuticals
Curated by ChEMBL
Rempex Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of tissue plasminogen activator (unknown origin) using GK-pNA as substrate preincubated for 10 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of dipeptidyl peptidase 7 (unknown origin) using H-Lys-Pro-AMC as substrate preincubated for 10 mins followed by substrate addition measur...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of neutrophil elastase (unknown origin) using MeOSuc-AAVP-AMC as substrate preincubated for 10 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of cathepsin A (unknown origin) using MCA-RPPGFSAFK-Dnp as substrate preincubated for 10 mins followed by substrate addition measured for ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of thrombin (unknown origin) using Benz-FVR-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of plasmin (unknown origin) using H-D-VLK-pNA as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of trypsin (unknown origin) using N-Bz-R-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of chymase (unknown origin) using Suc-AAPF-AMC as substrate preincubated for 10 mins followed by substrate addition measured for 30 mins b...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)