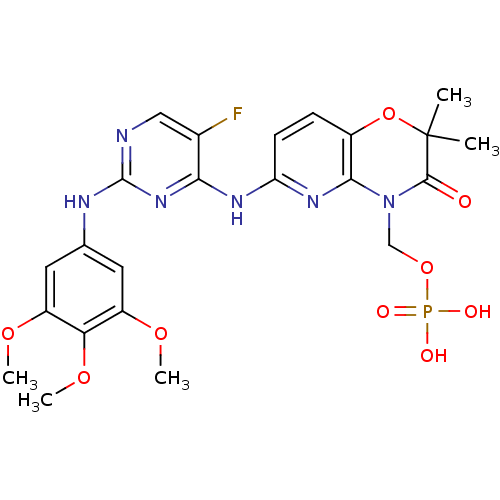
BDBM50431381 FOSTAMATINIB::R-788 Free acid::R-935788 Free acid
SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)N(COP(O)(O)=O)c4n3)n2)cc(OC)c1OC
InChI Key InChIKey=GKDRMWXFWHEQQT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50431381
Found 8 hits for monomerid = 50431381
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Hoffmann-La Roche
Curated by ChEMBL
Hoffmann-La Roche
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of human ERG channelMore data for this Ligand-Target Pair
Affinity DataIC50: 267nMAssay Description:Inhibition of SYK in human Ramos cellsMore data for this Ligand-Target Pair
TargetBromodomain testis-specific protein(Homo sapiens (Human))
University of Modena and Reggio Emilia
Curated by ChEMBL
University of Modena and Reggio Emilia
Curated by ChEMBL
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of human recombinant BRDTMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
Federal University of Parana
Curated by ChEMBL
Federal University of Parana
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human ABCG2 expressing membrane vesicles assessed inhibition of BCRP- mediated transport of [3H]estrone 3-sulfate for 2 mins using [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of SYK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of SYK in human whole bloodMore data for this Ligand-Target Pair